Type III interferons, IL-28 and IL-29, are increased in chronic HCV infection and induce myeloid dendritic cell-mediated FoxP3+ regulatory T cells
- PMID: 23071503
- PMCID: PMC3468613
- DOI: 10.1371/journal.pone.0044915
Type III interferons, IL-28 and IL-29, are increased in chronic HCV infection and induce myeloid dendritic cell-mediated FoxP3+ regulatory T cells
Abstract
Background & aims: Hepatitis C virus (HCV) is difficult to eradicate and type III interferons (IFN-λ, composed of IL-28A, IL-28B and IL-29) are novel therapeutic candidates. We hypothesized that IFN-λ have immunomodulatory effects in HCV- infected individuals.
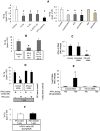
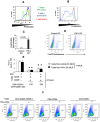
Materials and methods: We analyzed the expression of IFN-λ and its receptor (composed of IL-10R2 and IFN-λR subunits) in the blood and livers of patients with chronic (c)HCV infection compared to controls (those who cleared HCV by sustained virological response, SVR, and those with liver inflammation of non-viral origin, non-alcoholic steatohepatitis, NASH). We also compared the proliferative capacity of dendritic cells (DCs) obtained from healthy individuals and those with chronic HCV using a mixed leukocyte reaction combined with 3H-Td incorporation. In addition, the composition of the IFN-λ receptor (IFN-λR) on myeloid DCs, plasmacytoid DCs, PBMCs, and T cells was determined by FACS analysis.
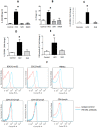
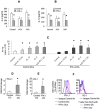
Results: We report that the expression of IFN-λ protein in serum and mRNA in liver is increased in cHCV patients, but not in those with HCV SVR or NASH, compared to controls. Liver level of IFN-λR mirrored the expression of serum IFN-λ and was higher in cHCV, compared to controls and HCV-SVR patients, suggesting that elevation of IFN-λ and IFN-λR are HCV-dependent. We further identified that innate immune cell populations expressed complete IFN-λ receptor. In vitro, recombinant IFN-λ promoted differentiation of monocyte-derived dendritic cells (DCs) into a phenotype with low T cell stimulatory capacity and high PD-L1 expression, which further promoted expansion of existing regulatory T cells. IFN-λ-DCs failed to induce de novo generation of regulatory T cells. The inhibitory capacity of IFN-λ-DCs was counteracted by recombinant IL-12 and by neutralization of the PD-1/PD-L1 system.
Conclusions: Our novel findings of the immunomodulatory effect of IFN-λ contribute to the understanding of the anti-inflammatory and/or anti-viral potential of IFN-λ in cHCV.
Conflict of interest statement
Figures
Similar articles
-
Human type 2 myeloid dendritic cells produce interferon-λ and amplify interferon-α in response to hepatitis C virus infection.Gastroenterology. 2013 Feb;144(2):414-425.e7. doi: 10.1053/j.gastro.2012.10.034. Epub 2012 Oct 23. Gastroenterology. 2013. PMID: 23089201 Free PMC article.
-
Human blood dendritic cell antigen 3 (BDCA3)(+) dendritic cells are a potent producer of interferon-λ in response to hepatitis C virus.Hepatology. 2013 May;57(5):1705-15. doi: 10.1002/hep.26182. Epub 2013 Mar 14. Hepatology. 2013. PMID: 23213063
-
Interferon-lambda serum levels in hepatitis C.J Hepatol. 2011 May;54(5):859-65. doi: 10.1016/j.jhep.2010.08.020. Epub 2010 Oct 23. J Hepatol. 2011. PMID: 21145813
-
Recent advances in the anti-HCV mechanisms of interferon.Acta Pharm Sin B. 2014 Aug;4(4):241-7. doi: 10.1016/j.apsb.2014.06.010. Epub 2014 Jul 14. Acta Pharm Sin B. 2014. PMID: 26579391 Free PMC article. Review.
-
Type III Interferons in Hepatitis C Virus Infection.Front Immunol. 2016 Dec 23;7:628. doi: 10.3389/fimmu.2016.00628. eCollection 2016. Front Immunol. 2016. PMID: 28066437 Free PMC article. Review.
Cited by
-
Host immune response in chronic hepatitis C infection: involvement of cytokines and inflammasomes.Rom J Morphol Embryol. 2020;61(1):33-43. doi: 10.47162/RJME.61.1.04. Rom J Morphol Embryol. 2020. PMID: 32747893 Free PMC article.
-
Human Regulatory T Cells From Umbilical Cord Blood Display Increased Repertoire Diversity and Lineage Stability Relative to Adult Peripheral Blood.Front Immunol. 2020 Apr 15;11:611. doi: 10.3389/fimmu.2020.00611. eCollection 2020. Front Immunol. 2020. PMID: 32351504 Free PMC article.
-
Differentially regulated gene expression associated with hepatitis C virus clearance.J Gen Virol. 2013 Mar;94(Pt 3):534-542. doi: 10.1099/vir.0.047738-0. Epub 2012 Nov 14. J Gen Virol. 2013. PMID: 23152368 Free PMC article.
-
IFN-λ Inhibits MiR-122 Transcription through a Stat3-HNF4α Inflammatory Feedback Loop in an IFN-α Resistant HCV Cell Culture System.PLoS One. 2015 Dec 11;10(12):e0141655. doi: 10.1371/journal.pone.0141655. eCollection 2015. PLoS One. 2015. PMID: 26657215 Free PMC article.
-
The Role of Type III Interferons in Hepatitis C Virus Infection and Therapy.J Immunol Res. 2017;2017:7232361. doi: 10.1155/2017/7232361. Epub 2017 Feb 1. J Immunol Res. 2017. PMID: 28255563 Free PMC article. Review.
References
-
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, et al. (2002) Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 347(13): 975–982. - PubMed
-
- Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, et al. (2006) Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 131(6): 1887–1898. - PubMed
-
- Uze G, Monneron D (2007) IL-28 and IL-29: newcomers to the interferon family. Biochimie 89: 729–734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

