Host generated siRNAs attenuate expression of serine protease gene in Myzus persicae
- PMID: 23071558
- PMCID: PMC3468595
- DOI: 10.1371/journal.pone.0046343
Host generated siRNAs attenuate expression of serine protease gene in Myzus persicae
Abstract
Background: Sap sucking hemipteran aphids damage diverse crop species. Although delivery of ds-RNA or siRNA through microinjection/feeding has been demonstrated, the efficacy of host-mediated delivery of aphid-specific dsRNA in developing aphid resistance has been far from being elucidated.
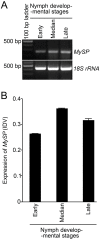
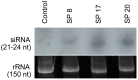
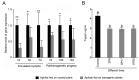
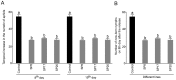
Methodology/principal findings: Transgenic Arabidopsis expressing ds-RNA of Myzus persicae serine protease (MySP) was developed that triggered the generation of corresponding siRNAs amenable for delivery to the feeding aphids. M. persicae when fed on the transgenic plants for different time intervals under controlled growth conditions resulted in a significant attenuation of the expression of MySP and a commensurate decline in gut protease activity. Although the survivability of these aphids was not affected, there was a noticeable decline in their fecundity resulting in a significant reduction in parthenogenetic population.
Conclusions/significance: The study highlighted the feasibility of developing host based RNAi-mediated resistance against hemipteran pest aphids.
Conflict of interest statement
Figures

References
-
- Oerke EC, Dehne HW, Schonbeck F, Weber A (1994) Crop production and crop protection–estimated losses in major food and cash crops. Amsterdam: Elsevier Science. 808 pp.
-
- McDougall P (2008) AgriService 2008. In: Saughland Phillips McDougall editor. Crop analysis. 532–768.
-
- Dedryver CA, Le Ralec A, Fabre F (2010) The conflicting relationships between aphids and men: A review of aphid damage and control strategies. CR Biol 333: 539–553. - PubMed
-
- Bhatia V, Uniyal PL, Bhattacharya RC (2011) Aphid resistance in Brassica crops: challenges, biotechnological progress and emerging possibilities. Biotechnol Adv 29: 879–888. - PubMed
-
- Nault LR (1997) Arthropod transmission of plant viruses: a new synthesis. Ann Entomol Soc Am 90(5): 521–541.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

