The nuclear receptor NR4A1 induces a form of cell death dependent on autophagy in mammalian cells
- PMID: 23071566
- PMCID: PMC3465341
- DOI: 10.1371/journal.pone.0046422
The nuclear receptor NR4A1 induces a form of cell death dependent on autophagy in mammalian cells
Erratum in
-
Correction: the nuclear receptor NR4A1 induces a form of cell death dependent on autophagy in mammalian cells.PLoS One. 2015 Mar 13;10(3):e0118718. doi: 10.1371/journal.pone.0118718. eCollection 2015. PLoS One. 2015. PMID: 25768666 Free PMC article. No abstract available.
Abstract
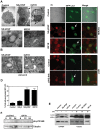
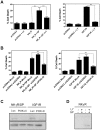
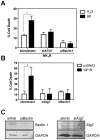
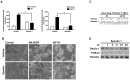
The control of cell death is a biological process essential for proper development, and for preventing devastating pathologies like cancer and neurodegeneration. On the other hand, autophagy regulation is essential for protein and organelle degradation, and its dysfunction is associated with overlapping pathologies like cancer and neurodegeneration, but also for microbial infection and aging. In the present report we show that two evolutionarily unrelated receptors--Neurokinin 1 Receptor (NK(1)R,) a G-protein coupled receptor, and Insulin-like Growth Factor 1 Receptor (IGF1R), a tyrosine kinase receptor--both induce non-apoptotic cell death with autophagic features and requiring the activity of the autophagic core machinery proteins PI3K-III, Beclin-1 and Atg7. Remarkably, this form of cell death occurs in apoptosis-competent cells. The signal transduction pathways engaged by these receptors both converged on the activation of the nuclear receptor NR4A1, which has previously been shown to play a critical role in some paradigms of apoptosis and in NK(1)R-induced cell death. The activity of NR4A1 was necessary for IGF1R-induced cell death, as well as for a canonical model of cell death by autophagy induced by the presence of a pan-caspase inhibitor, suggesting that NR4A1 is a general modulator of this kind of cell death. During cell death by autophagy, NR4A1 was transcriptionally competent, even though a fraction of it was present in the cytoplasm. Interestingly, NR4A1 interacts with the tumor suppressor p53 but not with Beclin-1 complex. Therefore the mechanism to promote cell death by autophagy might involve regulation of gene expression, as well as protein interactions. Understanding the molecular basis of autophagy and cell death mediation by NR4A1, should provide novel insights and targets for therapeutic intervention.
Conflict of interest statement
Figures


Similar articles
-
The nuclear receptor NR4A1 is regulated by SUMO modification to induce autophagic cell death.PLoS One. 2020 Mar 25;15(3):e0222072. doi: 10.1371/journal.pone.0222072. eCollection 2020. PLoS One. 2020. PMID: 32210435 Free PMC article.
-
Non-genomic effects of the NR4A1/Nur77/TR3/NGFIB orphan nuclear receptor.Steroids. 2015 Mar;95:1-6. doi: 10.1016/j.steroids.2014.12.020. Epub 2014 Dec 30. Steroids. 2015. PMID: 25555471 Review.
-
Regulation of autophagy by stress-responsive transcription factors.Semin Cancer Biol. 2013 Oct;23(5):310-22. doi: 10.1016/j.semcancer.2013.05.008. Epub 2013 May 30. Semin Cancer Biol. 2013. PMID: 23726895 Review.
-
NR4A1, 2, 3--an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis.Histol Histopathol. 2006 May;21(5):533-40. doi: 10.14670/HH-21.533. Histol Histopathol. 2006. PMID: 16493583 Review.
-
Orphan nuclear receptor NR4A1 regulates transforming growth factor-β signaling and fibrosis.Nat Med. 2015 Feb;21(2):150-8. doi: 10.1038/nm.3777. Epub 2015 Jan 12. Nat Med. 2015. PMID: 25581517
Cited by
-
Chymase mediates injury and mitochondrial damage in cardiomyocytes during acute ischemia/reperfusion in the dog.PLoS One. 2014 Apr 14;9(4):e94732. doi: 10.1371/journal.pone.0094732. eCollection 2014. PLoS One. 2014. PMID: 24733352 Free PMC article.
-
Glucocorticoids curtail stimuli-induced CREB phosphorylation in TRH neurons through interaction of the glucocorticoid receptor with the catalytic subunit of protein kinase A.Endocrine. 2017 Mar;55(3):861-871. doi: 10.1007/s12020-016-1223-z. Epub 2017 Jan 6. Endocrine. 2017. PMID: 28063130
-
Antagonist of the neurokinin-1 receptor curbs neuroinflammation in ex vivo and in vitro models of Lyme neuroborreliosis.J Neuroinflammation. 2015 Dec 30;12:243. doi: 10.1186/s12974-015-0453-y. J Neuroinflammation. 2015. PMID: 26714480 Free PMC article.
-
Correction: the nuclear receptor NR4A1 induces a form of cell death dependent on autophagy in mammalian cells.PLoS One. 2015 Mar 13;10(3):e0118718. doi: 10.1371/journal.pone.0118718. eCollection 2015. PLoS One. 2015. PMID: 25768666 Free PMC article. No abstract available.
-
Orphan Nuclear Receptor NR4A1 Binds a Novel Protein Interaction Site on Anti-apoptotic B Cell Lymphoma Gene 2 Family Proteins.J Biol Chem. 2016 Jul 1;291(27):14072-14084. doi: 10.1074/jbc.M116.715235. Epub 2016 Apr 19. J Biol Chem. 2016. PMID: 27129202 Free PMC article.
References
-
- Wingate AD, Arthur JS (2006) Post-translational control of Nur77. Biochem Soc Trans 34: 1107–1109. - PubMed
-
- Wu Q, Liu S, Ye XF, Huang ZW, Su WJ (2002) Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis 23: 1583–1592. - PubMed
-
- Winoto A, Littman DR (2002) Nuclear hormone receptors in T lymphocytes. Cell 109 Suppl: S57–66. - PubMed
-
- Liu W, Youn HD, Liu JO (2001) Thapsigargin-induced apoptosis involves Cabin1-MEF2-mediated induction of Nur77. Eur J Immunol 31: 1757–1764. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

