Of mice, birds, and men: the mouse ultrasonic song system has some features similar to humans and song-learning birds
- PMID: 23071596
- PMCID: PMC3468587
- DOI: 10.1371/journal.pone.0046610
Of mice, birds, and men: the mouse ultrasonic song system has some features similar to humans and song-learning birds
Abstract
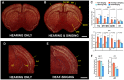
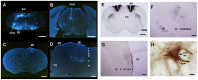
Humans and song-learning birds communicate acoustically using learned vocalizations. The characteristic features of this social communication behavior include vocal control by forebrain motor areas, a direct cortical projection to brainstem vocal motor neurons, and dependence on auditory feedback to develop and maintain learned vocalizations. These features have so far not been found in closely related primate and avian species that do not learn vocalizations. Male mice produce courtship ultrasonic vocalizations with acoustic features similar to songs of song-learning birds. However, it is assumed that mice lack a forebrain system for vocal modification and that their ultrasonic vocalizations are innate. Here we investigated the mouse song system and discovered that it includes a motor cortex region active during singing, that projects directly to brainstem vocal motor neurons and is necessary for keeping song more stereotyped and on pitch. We also discovered that male mice depend on auditory feedback to maintain some ultrasonic song features, and that sub-strains with differences in their songs can match each other's pitch when cross-housed under competitive social conditions. We conclude that male mice have some limited vocal modification abilities with at least some neuroanatomical features thought to be unique to humans and song-learning birds. To explain our findings, we propose a continuum hypothesis of vocal learning.
Conflict of interest statement
Figures




References
-
- Kroodsma DE, Konishi M (1991) A suboscine bird (eastern phoebe, Sayornis phoebe) develops normal song without auditory feedback. Anim Behav 42: 477–487.
-
- Janik VM, Slater PJB (1997) Vocal learning in mammals. Adv Stud Behav 26: 59–99.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

