Generation and characterization of human heme oxygenase-1 transgenic pigs
- PMID: 23071605
- PMCID: PMC3465346
- DOI: 10.1371/journal.pone.0046646
Generation and characterization of human heme oxygenase-1 transgenic pigs
Abstract
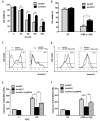
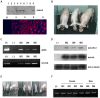
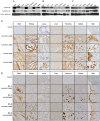
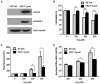
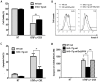
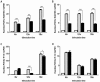
Xenotransplantation using transgenic pigs as an organ source is a promising strategy to overcome shortage of human organ for transplantation. Various genetic modifications have been tried to ameliorate xenograft rejection. In the present study we assessed effect of transgenic expression of human heme oxygenase-1 (hHO-1), an inducible protein capable of cytoprotection by scavenging reactive oxygen species and preventing apoptosis caused by cellular stress during inflammatory processes, in neonatal porcine islet-like cluster cells (NPCCs). Transduction of NPCCs with adenovirus containing hHO-1 gene significantly reduced apoptosis compared with the GFP-expressing adenovirus control after treatment with either hydrogen peroxide or hTNF-α and cycloheximide. These protective effects were diminished by co-treatment of hHO-1 antagonist, Zinc protoporphyrin IX. We also generated transgenic pigs expressing hHO-1 and analyzed expression and function of the transgene. Human HO-1 was expressed in most tissues, including the heart, kidney, lung, pancreas, spleen and skin, however, expression levels and patterns of the hHO-1 gene are not consistent in each organ. We isolate fibroblast from transgenic pigs to analyze protective effect of the hHO-1. As expected, fibroblasts derived from the hHO-1 transgenic pigs were significantly resistant to both hydrogen peroxide damage and hTNF-α and cycloheximide-mediated apoptosis when compared with wild-type fibroblasts. Furthermore, induction of RANTES in response to hTNF-α or LPS was significantly decreased in fibroblasts obtained from the hHO-1 transgenic pigs. These findings suggest that transgenic expression of hHO-1 can protect xenografts when exposed to oxidative stresses, especially from ischemia/reperfusion injury, and/or acute rejection mediated by cytokines. Accordingly, hHO-1 could be an important candidate molecule in a multi-transgenic pig strategy for xenotransplantation.
Conflict of interest statement
Figures
Similar articles
-
Generation of CMAHKO/GTKO/shTNFRI-Fc/HO-1 quadruple gene modified pigs.Transgenic Res. 2017 Aug;26(4):435-445. doi: 10.1007/s11248-017-0021-6. Epub 2017 May 28. Transgenic Res. 2017. PMID: 28553699
-
Production and characterization of soluble human TNFRI-Fc and human HO-1(HMOX1) transgenic pigs by using the F2A peptide.Transgenic Res. 2014 Jun;23(3):407-19. doi: 10.1007/s11248-013-9780-x. Epub 2014 Feb 5. Transgenic Res. 2014. PMID: 24497084
-
Transgenic expression of human heme oxygenase-1 in pigs confers resistance against xenograft rejection during ex vivo perfusion of porcine kidneys.Xenotransplantation. 2011 Nov-Dec;18(6):355-68. doi: 10.1111/j.1399-3089.2011.00674.x. Xenotransplantation. 2011. PMID: 22168142
-
The production of multi-transgenic pigs: update and perspectives for xenotransplantation.Transgenic Res. 2016 Jun;25(3):361-74. doi: 10.1007/s11248-016-9934-8. Epub 2016 Jan 28. Transgenic Res. 2016. PMID: 26820415 Review.
-
Genetic modification of pigs as organ donors for xenotransplantation.Mol Reprod Dev. 2010 Mar;77(3):209-21. doi: 10.1002/mrd.21127. Mol Reprod Dev. 2010. PMID: 19998476 Review.
Cited by
-
Heme-Oxygenase and Kidney Transplantation: A Potential for Target Therapy?Biomolecules. 2020 May 30;10(6):840. doi: 10.3390/biom10060840. Biomolecules. 2020. PMID: 32486245 Free PMC article. Review.
-
Production of Transgenic Pigs with an Introduced Missense Mutation of the Bone Morphogenetic Protein Receptor Type IB Gene Related to Prolificacy.Asian-Australas J Anim Sci. 2016 Jul;29(7):925-37. doi: 10.5713/ajas.15.0505. Epub 2015 Oct 29. Asian-Australas J Anim Sci. 2016. PMID: 26954151 Free PMC article.
-
Generation of CMAHKO/GTKO/shTNFRI-Fc/HO-1 quadruple gene modified pigs.Transgenic Res. 2017 Aug;26(4):435-445. doi: 10.1007/s11248-017-0021-6. Epub 2017 May 28. Transgenic Res. 2017. PMID: 28553699
-
Evidence for the important role of inflammation in xenotransplantation.J Inflamm (Lond). 2019 May 28;16:10. doi: 10.1186/s12950-019-0213-3. eCollection 2019. J Inflamm (Lond). 2019. PMID: 31148951 Free PMC article. Review.
-
Production of pigs expressing a transgene under the control of a tetracycline-inducible system.PLoS One. 2014 Jan 15;9(1):e86146. doi: 10.1371/journal.pone.0086146. eCollection 2014. PLoS One. 2014. PMID: 24454957 Free PMC article.
References
-
- Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, et al. (2002) Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295: 1089–1092. - PubMed
-
- Miyagawa S, Yamamoto A, Matsunami K, Wang D, Takama Y, et al. (2010) Complement regulation in the GalT KO era. Xenotransplantation 17: 11–25. - PubMed
-
- Charniot JC, Bonnefont-Rousselot D, Albertini JP, Zerhouni K, Dever S, et al. (2007) Oxidative stress implication in a new ex-vivo cardiac concordant xenotransplantation model. Free radical research 41: 911–918. - PubMed
-
- Li LP, Zhang L, Peng LP, Cheng L (2010) Heme oxygenase-1 is the candidate targeting for organ transplantation. Chinese medical journal 123: 2128–2134. - PubMed
-
- Ollinger R, Pratschke J (2010) Role of heme oxygenase-1 in transplantation. Transpl Int 23: 1071–1081. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

