Comparative study of human and mouse postsynaptic proteomes finds high compositional conservation and abundance differences for key synaptic proteins
- PMID: 23071613
- PMCID: PMC3465276
- DOI: 10.1371/journal.pone.0046683
Comparative study of human and mouse postsynaptic proteomes finds high compositional conservation and abundance differences for key synaptic proteins
Abstract
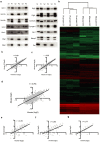
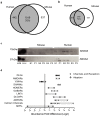
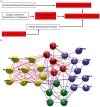
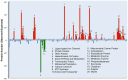
Direct comparison of protein components from human and mouse excitatory synapses is important for determining the suitability of mice as models of human brain disease and to understand the evolution of the mammalian brain. The postsynaptic density is a highly complex set of proteins organized into molecular networks that play a central role in behavior and disease. We report the first direct comparison of the proteome of triplicate isolates of mouse and human cortical postsynaptic densities. The mouse postsynaptic density comprised 1556 proteins and the human one 1461. A large compositional overlap was observed; more than 70% of human postsynaptic density proteins were also observed in the mouse postsynaptic density. Quantitative analysis of postsynaptic density components in both species indicates a broadly similar profile of abundance but also shows that there is higher abundance variation between species than within species. Well known components of this synaptic structure are generally more abundant in the mouse postsynaptic density. Significant inter-species abundance differences exist in some families of key postsynaptic density proteins including glutamatergic neurotransmitter receptors and adaptor proteins. Furthermore, we have identified a closely interacting set of molecules enriched in the human postsynaptic density that could be involved in dendrite and spine structural plasticity. Understanding synapse proteome diversity within and between species will be important to further our understanding of brain complexity and disease.
Conflict of interest statement
Figures

References
-
- Bayés A, Grant SG (2009) Neuroproteomics: understanding the molecular organization and complexity of the brain. Nat Rev Neurosci 10: 635–646. - PubMed
-
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG (2000) Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nature neuroscience 3: 661–669. - PubMed
-
- Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, et al. (2006) Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J Neurochem 97 Suppl 1: 16–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

