Actin recruitment to the Chlamydia inclusion is spatiotemporally regulated by a mechanism that requires host and bacterial factors
- PMID: 23071671
- PMCID: PMC3469565
- DOI: 10.1371/journal.pone.0046949
Actin recruitment to the Chlamydia inclusion is spatiotemporally regulated by a mechanism that requires host and bacterial factors
Abstract
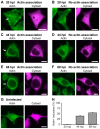
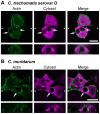
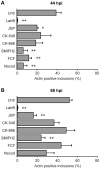
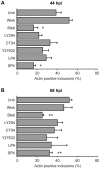
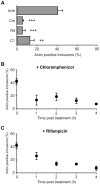
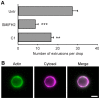
The ability to exit host cells at the end of their developmental growth is a critical step for the intracellular bacterium Chlamydia. One exit strategy, extrusion, is mediated by host signaling pathways involved with actin polymerization. Here, we show that actin is recruited to the chlamydial inclusion as a late event, occurring after 20 hours post-infection (hpi) and only within a subpopulation of cells. This event increases significantly in prevalence and extent from 20 to 68 hpi, and actin coats strongly correlated with extrusions. In contrast to what has been reported for other intracellular pathogens, actin nucleation on Chlamydia inclusions did not 'flash', but rather exhibited moderate depolymerization dynamics. By using small molecule agents to selectively disrupt host signaling pathways involved with actin nucleation, modulate actin polymerization dynamics and also to disable the synthesis and secretion of chlamydial proteins, we further show that host and bacterial proteins are required for actin coat formation. Transient disruption of either host or bacterial signaling pathways resulted in rapid loss of coats in all infected cells and a reduction in extrusion formation. Inhibition of Chlamydia type III secretion also resulted in rapid loss of actin association on inclusions, thus implicating chlamydial effector proteins(s) as being central factors for engaging with host actin nucleating factors, such as formins. In conclusion, our data illuminate the host and bacterial driven process by which a dense actin matrix is dynamically nucleated and maintained on the Chlamydia inclusion. This late stage event is not ubiquitous for all infected cells in a population, and escalates in prevalence and extent throughout the developmental cycle of Chlamydia, culminating with their exit from the host cell by extrusion. The initiation of actin recruitment by Chlamydia appears to be novel, and may serve as an upstream determinant of the extrusion mechanism.
Conflict of interest statement
Figures



References
-
- Stamm WE (1999) Chlamydia trachomatis infections: progress and problems. J Infect Dis 179 Suppl 2: S380–S383. - PubMed
-
- Schachter J (1999) Infection and disease epidemiology. In: Stephens RS, editor. Chlamydia: Intracellular Biology, Pathogenesis, and Immunity. American Society for Microbiology Press. pp. 139–169.
-
- Mathers C, Fat DM (2008) The global burden of disease: 2004 update. Geneva: World Health Organization.
-
- Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, et al. (2010) Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 201 Suppl 2: S134–S155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

