Atomic force microscopy analysis of the Acinetobacter baumannii bacteriophage AP22 lytic cycle
- PMID: 23071792
- PMCID: PMC3469531
- DOI: 10.1371/journal.pone.0047348
Atomic force microscopy analysis of the Acinetobacter baumannii bacteriophage AP22 lytic cycle
Abstract
Background: Acinetobacter baumannii is known for its ability to develop resistance to the major groups of antibiotics, form biofilms, and survive for long periods in hospital environments. The prevalence of infections caused by multidrug-resistant A. baumannii is a significant problem for the modern health care system, and application of lytic bacteriophages for controlling this pathogen may become a solution.
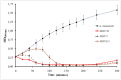
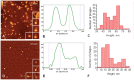
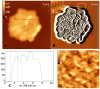
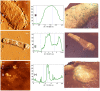
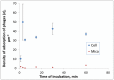
Methodology/principal findings: In this study, using atomic force microscopy (AFM) and microbiological assessment we have investigated A. baumannii bacteriophage AP22, which has been recently described. AFM has revealed the morphology of bacteriophage AP22, adsorbed on the surfaces of mica, graphite and host bacterial cells. Besides, morphological changes of bacteriophage AP22-infected A. baumannii cells were characterized at different stages of the lytic cycle, from phage adsorption to the cell lysis. The phage latent period, estimated from AFM was in good agreement with that obtained by microbiological methods (40 min). Bacteriophage AP22, whose head diameter is 62±1 nm and tail length is 88±9 nm, was shown to disperse A. baumannii aggregates and adsorb to the bacterial surface right from the first minute of their mutual incubation at 37°C.
Conclusions/significance: High rate of bacteriophage AP22 specific adsorption and its ability to disperse bacterial aggregates make this phage very promising for biomedical antimicrobial applications. Complementing microbiological results with AFM data, we demonstrate an effective approach, which allows not only comparing independently obtained characteristics of the lytic cycle but also visualizing the infection process.
Conflict of interest statement
Figures


Similar articles
-
Characterization, sequencing and comparative genomic analysis of vB_AbaM-IME-AB2, a novel lytic bacteriophage that infects multidrug-resistant Acinetobacter baumannii clinical isolates.BMC Microbiol. 2014 Jul 5;14:181. doi: 10.1186/1471-2180-14-181. BMC Microbiol. 2014. PMID: 24996449 Free PMC article.
-
Isolation and characterization of wide host range lytic bacteriophage AP22 infecting Acinetobacter baumannii.FEMS Microbiol Lett. 2012 Jul;332(1):40-6. doi: 10.1111/j.1574-6968.2012.02573.x. Epub 2012 May 8. FEMS Microbiol Lett. 2012. PMID: 22506502
-
Potential of a lytic bacteriophage to disrupt Acinetobacter baumannii biofilms in vitro.Future Microbiol. 2016 Oct;11:1383-1393. doi: 10.2217/fmb-2016-0104. Epub 2016 Aug 18. Future Microbiol. 2016. PMID: 27538011
-
Specific and Selective Bacteriophages in the Fight against Multidrug-resistant Acinetobacter baumannii.Virol Sin. 2019 Aug;34(4):347-357. doi: 10.1007/s12250-019-00125-0. Epub 2019 May 15. Virol Sin. 2019. PMID: 31093881 Free PMC article. Review.
-
Acinetobacter baumannii: More ways to die.Microbiol Res. 2022 Aug;261:127069. doi: 10.1016/j.micres.2022.127069. Epub 2022 May 13. Microbiol Res. 2022. PMID: 35623161 Review.
Cited by
-
Phage Therapy as a Focused Management Strategy in Aquaculture.Int J Mol Sci. 2021 Sep 28;22(19):10436. doi: 10.3390/ijms221910436. Int J Mol Sci. 2021. PMID: 34638776 Free PMC article. Review.
-
Real-time monitoring by interferometric light microscopy of phage suspensions for personalised phage therapy.Sci Rep. 2024 Dec 30;14(1):31629. doi: 10.1038/s41598-024-79478-w. Sci Rep. 2024. PMID: 39738265 Free PMC article.
-
Characterization and genome sequencing of phage Abp1, a new phiKMV-like virus infecting multidrug-resistant Acinetobacter baumannii.Curr Microbiol. 2013 Jun;66(6):535-43. doi: 10.1007/s00284-013-0308-7. Epub 2013 Jan 18. Curr Microbiol. 2013. PMID: 23328903
-
The Use of Atomic Force Microscopy for 3D Analysis of Nucleic Acid Hybridization on Microarrays.Acta Naturae. 2015 Apr-Jun;7(2):108-14. Acta Naturae. 2015. PMID: 26085952 Free PMC article.
-
Bacteriophage ZCSE2 is a Potent Antimicrobial Against Salmonella enterica Serovars: Ultrastructure, Genomics and Efficacy.Viruses. 2020 Apr 9;12(4):424. doi: 10.3390/v12040424. Viruses. 2020. PMID: 32283768 Free PMC article.
References
-
- Towner KJ (2009) Acinetobacter: an old friend, but a new enemy. J Hosp Infect 73(4): 355–63. - PubMed
-
- Webster CA, Crow M, Humphreys H, Towner KS (1998) Surveillance of an adult intensive care unit for a long-term persistence of a multiresistant strain of Acinetobacter baumannii . Eur J Clin Microbiol Infect Dis 17: 171–176. - PubMed
-
- de Oliveira AC, Damasceno QS (2010) Surfaces of the hospital environment as possible deposits of resistant bacteria: a review. Rev Esc Enferm USP 44: 1118–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

