The inhibitor of volume-regulated anion channels DCPIB activates TREK potassium channels in cultured astrocytes
- PMID: 23072356
- PMCID: PMC3594680
- DOI: 10.1111/bph.12011
The inhibitor of volume-regulated anion channels DCPIB activates TREK potassium channels in cultured astrocytes
Abstract
Background and purpose: The ethacrynic acid derivative, 4-(2-butyl-6,7-dichlor-2-cyclopentylindan-1-on-5-yl) oxobutyric acid (DCPIB) is considered to be a specific and potent inhibitor of volume-regulated anion channels (VRACs). In the CNS, DCPIB was shown to be neuroprotective through mechanisms principally associated to its action on VRACs. We hypothesized that DCPIB could also regulate the activity of other astroglial channels involved in cell volume homeostasis.
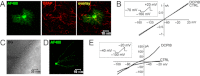
Experimental approach: Experiments were performed in rat cortical astrocytes in primary culture and in hippocampal astrocytes in situ. The effect of DCPIB was evaluated by patch-clamp electrophysiology and immunocytochemical techniques. Results were verified by comparative analysis with recombinant channels expressed in COS-7 cells.
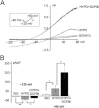
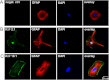
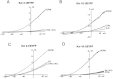
Key results: In cultured astrocytes, DCPIB promoted the activation of a K(+) conductance mediated by two-pore-domain K(+) (K(2P) ) channels. The DCPIB effect occluded that of arachidonic acid, which activates K(2P) channels K(2P) 2.1 (TREK-1) and K(2P) 10.1 (TREK-2) in cultured astrocytes. Immunocytochemical analysis suggests that cultured astrocytes express K(2P) 2.1 and K(2P) 10.1 proteins. Moreover, DCPIB opened recombinant K(2P) 2.1 and K(2P) 10.1 expressed in heterologous system. In brain slices, DCPIB did not augment the large background K(+) conductance in hippocampal astrocytes, but caused an increment in basal K(+) current of neurons.
Conclusion and implications: Our results indicate that the neuroprotective effect of DCPIB could be due, at least in part, to activation of TREK channels. DCPIB could be used as template to build new pharmacological tools able to increase background K(+) conductance in astroglia and neuronal cells.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures



Similar articles
-
DCPIB, an Inhibitor of Volume-Regulated Anion Channels, Distinctly Modulates K2P Channels.ACS Chem Neurosci. 2019 Jun 19;10(6):2786-2793. doi: 10.1021/acschemneuro.9b00010. Epub 2019 Apr 17. ACS Chem Neurosci. 2019. PMID: 30935201
-
DCPIB, a potent volume-regulated anion channel antagonist, attenuates microglia-mediated inflammatory response and neuronal injury following focal cerebral ischemia.Brain Res. 2014 Jan 13;1542:176-85. doi: 10.1016/j.brainres.2013.10.026. Epub 2013 Nov 1. Brain Res. 2014. PMID: 24189520
-
TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices.J Neurosci. 2009 Jul 1;29(26):8551-64. doi: 10.1523/JNEUROSCI.5784-08.2009. J Neurosci. 2009. PMID: 19571146 Free PMC article.
-
The TREK K2P channels and their role in general anaesthesia and neuroprotection.Trends Pharmacol Sci. 2004 Nov;25(11):601-8. doi: 10.1016/j.tips.2004.09.003. Trends Pharmacol Sci. 2004. PMID: 15491783 Review.
-
Patents related to therapeutic activation of K(ATP) and K(2P) potassium channels for neuroprotection: ischemic/hypoxic/anoxic injury and general anesthetics.Expert Opin Ther Pat. 2009 Apr;19(4):433-60. doi: 10.1517/13543770902765151. Expert Opin Ther Pat. 2009. PMID: 19441925 Review.
Cited by
-
Two-pore Domain Potassium Channels in Astrocytes.Exp Neurobiol. 2016 Oct;25(5):222-232. doi: 10.5607/en.2016.25.5.222. Epub 2016 Oct 26. Exp Neurobiol. 2016. PMID: 27790056 Free PMC article. Review.
-
Astrocytic modulation of potassium under seizures.Neural Regen Res. 2020 Jun;15(6):980-987. doi: 10.4103/1673-5374.270295. Neural Regen Res. 2020. PMID: 31823867 Free PMC article. Review.
-
Physiological Functions of the Volume-Regulated Anion Channel VRAC/LRRC8 and the Proton-Activated Chloride Channel ASOR/TMEM206.Handb Exp Pharmacol. 2024;283:181-218. doi: 10.1007/164_2023_673. Handb Exp Pharmacol. 2024. PMID: 37468723
-
LRRC8/VRAC anion channels enhance β-cell glucose sensing and insulin secretion.Nat Commun. 2018 May 17;9(1):1974. doi: 10.1038/s41467-018-04353-y. Nat Commun. 2018. PMID: 29773801 Free PMC article.
-
Syringin inhibits endogenous volume regulated anion channel currents of HEK293 cells in hypotonic circumstances.Pharmacol Res Perspect. 2023 Jun;11(3):e01105. doi: 10.1002/prp2.1105. Pharmacol Res Perspect. 2023. PMID: 37278329 Free PMC article.
References
-
- Aller MI, Wisden W. Changes in expression of some two-pore domain potassium channel genes (KCNK) in selected brain regions of developing mice. Neuroscience. 2008;151:1154–1172. - PubMed
-
- Benesova J, Hock M, Butenko O, Prajerova I, Anderova M, Chvatal A. Quantification of astrocyte volume changes during ischemia in situ reveals two populations of astrocytes in the cortex of GFAP/EGFP mice. J Neurosci Res. 2009;87:96–111. - PubMed
-
- Benfenati V, Caprini M, Nicchia GP, Rossi A, Dovizio M, Cervetto C, et al. Carbenoxolone inhibits volume-regulated anion conductance in cultured rat cortical astroglia. Channels. 2009;3:323–336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

