The nervous system of Isodiametra pulchra (Acoela) with a discussion on the neuroanatomy of the Xenacoelomorpha and its evolutionary implications
- PMID: 23072457
- PMCID: PMC3488495
- DOI: 10.1186/1742-9994-9-27
The nervous system of Isodiametra pulchra (Acoela) with a discussion on the neuroanatomy of the Xenacoelomorpha and its evolutionary implications
Abstract
Introduction: Acoels are microscopic marine worms that have become the focus of renewed debate and research due to their placement at the base of the Bilateria by molecular phylogenies. To date, Isodiametra pulchra is the most promising "model acoel" as it can be cultured and gene knockdown can be performed with double-stranded RNA. Despite its well-known morphology data on the nervous system are scarce. Therefore we examined this organ using various microscopic techniques, including histology, conventional histochemistry, electron microscopy, and immunocytochemistry in combination with CLSM and discuss our results in light of recently established phylogenies.
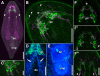
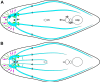
Results: The nervous system of Isodiametra pulchra consists of a bilobed brain with a dorsal posterior commissure, a frontal ring and tracts, four pairs of longitudinal neurite bundles, as well as a supramuscular and submuscular plexus. Serotonin-like immunoreactivity (SLI) is displayed in parts of the brain, the longitudinal neurite bundles and a large part of the supramuscular plexus, while FMRFamide-like immunoreactivity (RFLI) is displayed in parts of the brain and a distinct set of neurons, the longitudinal neurite bundles and the submuscular plexus. Despite this overlap SLI and RFLI are never colocalized. Most remarkable though is the presence of a distinct functional neuro-muscular system consisting of the statocyst, tracts, motor neurons and inner muscles, as well as the presence of various muscles that differ with regard to their ultrastructure and innervation.
Conclusions: The nervous system of Isodiametra pulchra consists of an insunk, bilobed brain, a peripheral part for perception and innervation of the smooth body-wall musculature as well as tracts and motor neurons that together with pseudostriated inner muscles are responsible for steering and quick movements. The insunk, bilobed brains with two to three commissures found in numerous acoels are homologous and evolved from a ring-commissural brain that was present in the stem species of acoelomorphs. The acoelomorph brain is bipartite, consisting of a Six3/6-dependend animal pole nervous system that persists throughout adulthood and an axial nervous system that does not develop by exhibiting a staggered pattern of conserved regulatory genes as in other bilaterians but by a nested pattern of these genes. This indicates that acoelomorphs stem from an ancestor with a simple brain or with a biphasic life cycle.
Figures








Similar articles
-
SALMFamide2 and serotonin immunoreactivity in the nervous system of some acoels (Xenacoelomorpha).J Morphol. 2018 May;279(5):589-597. doi: 10.1002/jmor.20794. Epub 2018 Feb 1. J Morphol. 2018. PMID: 29388261 Free PMC article.
-
Inferring the ancestral function of the posterior Hox gene within the bilateria: controlling the maintenance of reproductive structures, the musculature and the nervous system in the acoel flatworm Isodiametra pulchra.Evol Dev. 2010 May-Jun;12(3):258-66. doi: 10.1111/j.1525-142X.2010.00411.x. Evol Dev. 2010. PMID: 20565536
-
Characterization of the stem cell system of the acoel Isodiametra pulchra.BMC Dev Biol. 2009 Dec 18;9:69. doi: 10.1186/1471-213X-9-69. BMC Dev Biol. 2009. PMID: 20017953 Free PMC article.
-
Identification of interstitial cells of Cajal. Significance for studies of human small intestine and colon.Dan Med Bull. 1994 Jun;41(3):275-93. Dan Med Bull. 1994. PMID: 7924459 Review.
-
When does a ganglion become a brain? Evolutionary origin of the central nervous system.Semin Pediatr Neurol. 2002 Dec;9(4):240-53. doi: 10.1053/spen.2002.32502. Semin Pediatr Neurol. 2002. PMID: 12523550 Review.
Cited by
-
Physiology of Astroglia.Physiol Rev. 2018 Jan 1;98(1):239-389. doi: 10.1152/physrev.00042.2016. Physiol Rev. 2018. PMID: 29351512 Free PMC article. Review.
-
Peripheral and central employment of acid-sensing ion channels during early bilaterian evolution.Elife. 2023 Feb 23;12:e81613. doi: 10.7554/eLife.81613. Elife. 2023. PMID: 36821351 Free PMC article.
-
The Acoel nervous system: morphology and development.Neural Dev. 2024 Jun 21;19(1):9. doi: 10.1186/s13064-024-00187-1. Neural Dev. 2024. PMID: 38907301 Free PMC article. Review.
-
SALMFamide2 and serotonin immunoreactivity in the nervous system of some acoels (Xenacoelomorpha).J Morphol. 2018 May;279(5):589-597. doi: 10.1002/jmor.20794. Epub 2018 Feb 1. J Morphol. 2018. PMID: 29388261 Free PMC article.
-
Structure of putative epidermal sensory receptors in an acoel flatworm, Praesagittifera naikaiensis.Cell Tissue Res. 2024 Mar;395(3):299-311. doi: 10.1007/s00441-024-03865-y. Epub 2024 Feb 2. Cell Tissue Res. 2024. PMID: 38305882 Free PMC article.
References
-
- Hyman LH. The Invertebrates: Platyhelminthes and Rhynchocoela. The acoelomate Bilateria. Volume 2. McGraw Hill Book Co, New York; 1951.
-
- Westblad E. Studien über Skandinavische Turbellaria Acoela. 5. Arkiv för Zoologi. 1948;41A(7):1–82.
-
- Raikova OI, Reuter M, Kotikova EA, Gustafsson MKS. A commissural brain! The pattern of 5-HT immunoreactivity in Acoela (Plathelminthes) [Platyhelminthes] Zoomorphology. 1998;118(2):69–77. doi: 10.1007/s004350050058. - DOI
-
- Reuter M, Raikova OI, Gustafsson MKS. Patterns in the nervous and muscle systems in lower flatworms. Belgian J Zool. 2001;131:47–53.

