Local modulation of cystic fibrosis conductance regulator: cytoskeleton and compartmentalized cAMP signalling
- PMID: 23072488
- PMCID: PMC3632233
- DOI: 10.1111/bph.12017
Local modulation of cystic fibrosis conductance regulator: cytoskeleton and compartmentalized cAMP signalling
Abstract
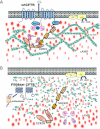
The cystic fibrosis conductance regulator (CFTR) is a cAMP-regulated Cl(-) channel expressed predominantly at the apical membrane of secreting epithelial cells. Mutations in the CFTR gene lead to cystic fibrosis, the most frequent genetic disease in the Caucasian population. The most common mutation, a deletion of phenylalanine at position 508 (F508del), impairs CFTR folding and chloride channel function. Although an intense effort is under way to identify compounds that target the F508del CFTR structural defect and promote its expression and stability at the plasma membrane, so far their clinical efficacy has proven to be poor, highlighting the necessity to better understand the molecular mechanism of CFTR regulation and of the pathogenesis of the disease. Accumulating evidence suggests that the inclusion of the CFTR in macromolecular complexes and its interaction with the cortical cytoskeleton may play a key role in fine-tuning the regulation of channel function. Here we review some recent findings that support a critical role for protein-protein interactions involving CFTR and for the cytoskeleton in promoting local control of channel activity. These findings indicate that compounds that rescue and stabilize CFTR at the apical membrane may not be sufficient to restore its function unless the appropriate intracellular milieu is also reconstituted.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures
Similar articles
-
Correctors of mutant CFTR enhance subcortical cAMP-PKA signaling through modulating ezrin phosphorylation and cytoskeleton organization.J Cell Sci. 2016 Mar 15;129(6):1128-40. doi: 10.1242/jcs.177907. Epub 2016 Jan 28. J Cell Sci. 2016. PMID: 26823603
-
Two Small Molecules Restore Stability to a Subpopulation of the Cystic Fibrosis Transmembrane Conductance Regulator with the Predominant Disease-causing Mutation.J Biol Chem. 2017 Mar 3;292(9):3706-3719. doi: 10.1074/jbc.M116.751537. Epub 2017 Jan 13. J Biol Chem. 2017. PMID: 28087700 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator (CFTR) and renal function.Wien Klin Wochenschr. 1997 Jun 27;109(12-13):457-64. Wien Klin Wochenschr. 1997. PMID: 9261986 Review.
-
Resveratrol rescues cAMP-dependent anionic transport in the cystic fibrosis pancreatic cell line CFPAC1.Br J Pharmacol. 2011 Jun;163(4):876-86. doi: 10.1111/j.1476-5381.2011.01289.x. Br J Pharmacol. 2011. PMID: 21366549 Free PMC article.
-
Targeting F508del-CFTR to develop rational new therapies for cystic fibrosis.Acta Pharmacol Sin. 2011 Jun;32(6):693-701. doi: 10.1038/aps.2011.71. Acta Pharmacol Sin. 2011. PMID: 21642944 Free PMC article. Review.
Cited by
-
CFTR and sphingolipids mediate hypoxic pulmonary vasoconstriction.Proc Natl Acad Sci U S A. 2015 Mar 31;112(13):E1614-23. doi: 10.1073/pnas.1421190112. Epub 2015 Mar 17. Proc Natl Acad Sci U S A. 2015. PMID: 25829545 Free PMC article.
-
The Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Uses its C-Terminus to Regulate the A2B Adenosine Receptor.Sci Rep. 2016 Jun 9;6:27390. doi: 10.1038/srep27390. Sci Rep. 2016. PMID: 27278076 Free PMC article.
-
Tobacco Smoke Constituents Trigger Cytoplasmic Calcium Release.Appl In Vitro Toxicol. 2017 Jun 1;3(2):193-198. doi: 10.1089/aivt.2016.0039. Appl In Vitro Toxicol. 2017. PMID: 28620626 Free PMC article.
-
CFTR, Cell Junctions and the Cytoskeleton.Int J Mol Sci. 2022 Feb 28;23(5):2688. doi: 10.3390/ijms23052688. Int J Mol Sci. 2022. PMID: 35269829 Free PMC article. Review.
-
Evidence for a causal link between adaptor protein PDZK1 downregulation and Na⁺/H⁺ exchanger NHE3 dysfunction in human and murine colitis.Pflugers Arch. 2015 Aug;467(8):1795-807. doi: 10.1007/s00424-014-1608-x. Epub 2014 Oct 2. Pflugers Arch. 2015. PMID: 25271043 Free PMC article.
References
-
- Barnes AP, Livera G, Huang P, Sun C, O'Neal WK, Conti M, et al. Phosphodiesterase 4D forms a cAMP diffusion barrier at the apical membrane of the airway epithelium. J Biol Chem. 2005;280:7997–8003. - PubMed
-
- Becq F, Mall MA, Sheppard DN, Conese M, Zegarra-Moran O. Pharmacological therapy for cystic fibrosis: from bench to bedside. J Cyst Fibros. 2011;10(Suppl. 2):S129–S145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

