Nonredundant regulation of rice arbuscular mycorrhizal symbiosis by two members of the phosphate transporter1 gene family
- PMID: 23073651
- PMCID: PMC3517247
- DOI: 10.1105/tpc.112.104901
Nonredundant regulation of rice arbuscular mycorrhizal symbiosis by two members of the phosphate transporter1 gene family
Abstract
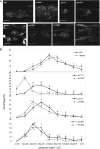
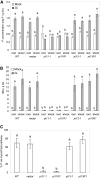
Pi acquisition of crops via arbuscular mycorrhizal (AM) symbiosis is becoming increasingly important due to limited high-grade rock Pi reserves and a demand for environmentally sustainable agriculture. Here, we show that 70% of the overall Pi acquired by rice (Oryza sativa) is delivered via the symbiotic route. To better understand this pathway, we combined genetic, molecular, and physiological approaches to determine the specific functions of two symbiosis-specific members of the PHOSPHATE TRANSPORTER1 (PHT1) gene family from rice, ORYsa;PHT1;11 (PT11) and ORYsa;PHT1;13 (PT13). The PT11 lineage of proteins from mono- and dicotyledons is most closely related to homologs from the ancient moss, indicating an early evolutionary origin. By contrast, PT13 arose in the Poaceae, suggesting that grasses acquired a particular strategy for the acquisition of symbiotic Pi. Surprisingly, mutations in either PT11 or PT13 affected the development of the symbiosis, demonstrating that both genes are important for AM symbiosis. For symbiotic Pi uptake, however, only PT11 is necessary and sufficient. Consequently, our results demonstrate that mycorrhizal rice depends on the AM symbiosis to satisfy its Pi demands, which is mediated by a single functional Pi transporter, PT11.
Figures







References
-
- Ai P., Sun S., Zhao J., Fan X., Xin W., Guo Q., Yu L., Shen Q., Wu P., Miller A.J., Xu G. (2009). Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J. 57: 798–809 - PubMed
-
- Araki R., Hasegawa H. (2006). Expression of rice (Oryza sativa L.) genes involved in high-affinity nitrate transport during the period of nitrate induction. Breed. Sci. 56: 295–302
-
- Barea J. (1991). Vesicular-arbuscular mycorrhizae as modifiers of soil feritlity. In Advances in Soil Science 15, B. Stewart, ed (New York: Springer), pp. 1–40
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

