A Janus tale of two active high mobility group box 1 (HMGB1) redox states
- PMID: 23073660
- PMCID: PMC3533642
- DOI: 10.2119/molmed.2012.00314
A Janus tale of two active high mobility group box 1 (HMGB1) redox states
Abstract
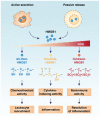
High mobility group box 1 (HMGB1), the prototypic damage-associated molecular pattern molecule, is released at sites of inflammation and/or tissue damage. There, it promotes cytokine production and chemokine production/cell migration. New work shows that the redox status of HMGB1 distinguishes its cytokine-inducing and chemokine activity. Reduced all-thiol-HMGB1 has sole chemokine activity, whereas disulfide-HMGB1 has only cytokine activity, and oxidized, denatured HMGB1 has neither. Autophagy (programmed cell survival) and apoptosis (programmed cell death) have been implicated in controlling both innate and adaptive immune functions. Reduced HMGB1 protein promotes autophagy, whereas oxidized HMGB1 promotes apoptosis. Thus, the differential activity of HMGB1 in immunity, inflammation and cell death depends on the cellular redox status within tissues.
Figures
References
-
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. - PubMed
-
- Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving auto-immune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. - PubMed
-
- Lotze MT, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

