Toll-like receptor 2 ligands promote microglial cell death by inducing autophagy
- PMID: 23073832
- PMCID: PMC3528320
- DOI: 10.1096/fj.12-214312
Toll-like receptor 2 ligands promote microglial cell death by inducing autophagy
Abstract
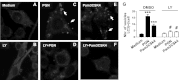
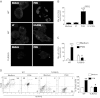
Microglial cells are phagocytes in the central nervous system (CNS) and become activated in pathological conditions, resulting in microgliosis, manifested by increased cell numbers and inflammation in the affected regions. Thus, controlling microgliosis is important to prevent pathological damage to the brain. Here, we evaluated the contribution of Toll-like receptor 2 (TLR2) to microglial survival. We observed that activation of microglial cells with peptidoglycan (PGN) from Staphylococcus aureus and other TLR2 ligands results in cell activation followed by the induction of autophagy and autophagy-dependent cell death. In C57BL/6J mice, intracerebral injection of PGN increased the autophagy of microglial cells and reduced the microglial/macrophage cell number in brain parenchyma. Our results demonstrate a novel role of TLRs in the regulation of microglial cell activation and survival, which are important for the control of microgliosis and associated inflammatory responses in the CNS.
Figures







References
-
- Mehrpour M., Esclatine A., Beau I., Codogno P. (2010) Overview of macroautophagy regulation in mammalian cells. Cell Res. 20, 748–762 - PubMed
-
- Bursch W., Karwan A., Mayer M., Dornetshuber J., Frohwein U., Schulte-Hermann R., Fazi B., Di Sano F., Piredda L., Piacentini M., Petrovski G., Fesus L., Gerner C. (2008) Cell death and autophagy: cytokines, drugs, and nutritional factors. Toxicology 254, 147–157 - PubMed
-
- Maiuri M. C., Zalckvar E., Kimchi A., Kroemer G. (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 8, 741–752 - PubMed
-
- Nakagawa I., Amano A., Mizushima N., Yamamoto A., Yamaguchi H., Kamimoto T., Nara A., Funao J., Nakata M., Tsuda K., Hamada S., Yoshimori T. (2004) Autophagy defends cells against invading group A Streptococcus. Science 306, 1037–1040 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

