Reconciling the stereochemical course of nucleopalladation with the development of enantioselective wacker-type cyclizations
- PMID: 23074039
- PMCID: PMC3492954
- DOI: 10.1002/anie.201206702
Reconciling the stereochemical course of nucleopalladation with the development of enantioselective wacker-type cyclizations
Abstract
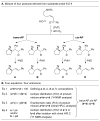
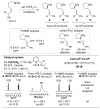
A novel stereochemical substrate probe was used to assess the factors that affect the stereochemical course of nucleopalladation (cis vs. trans) in the context of an enantioselective Wacker-type reaction. We demonstrate that the enantioselectivity correlates directly with the nucleopalladation pathway, and both the neutral-donor and anionic ligands on palladium are capable of controlling selectivity for cis or trans nucleopalladation.
Figures

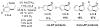

References
-
- Henry PM. Palladium Catalyzed Oxidation of Hydrocarbons. Vol. 2. D. Reidel Publishing Co; Dordrecht, The Netherlands: 1980.
-
- Zeni G, Larock RC. Chem Rev. 2004;104:2285–2309. - PubMed
- Beccalli EM, Broggini G, Martinelli M, Sottocornola S. Chem Rev. 2007;107:5318–5365. - PubMed
- Minatti A, Muñiz K. Chem Soc Rev. 2007;36:1142–1152. - PubMed
- Kotov V, Scarborough CC, Stahl SS. Inorg Chem. 2007;46:1910–1923. - PubMed
- Jensen KH, Sigman MS. Org Biomol Chem. 2008;6:4083–4088. - PMC - PubMed
-
-
For leading primary references, see: Hosokawa T, Uno T, Inui S, Murahashi SI. J Am Chem Soc. 1981;103:2318–2323.Uozomi Y, Kato K, Hayashi T. J Am Chem Soc. 1997;119:5063–5064.Uozomi Y, Kato K, Hayashi T. J Org Chem. 1998;63:5071–5075.Arai MA, Kuraishi M, Arai T, Sasai H. J Am Chem Soc. 2001;123:2907–2908.Trend RM, Ramtohul YK, Ferreira EM, Stoltz BM. Angew Chem Int Ed. 2003;42:2892–2895.Angew Chem. 2003;115:2998–3001.Hayashi T, Yamasaki K, Mimura M, Uozumi Y. J Am Chem Soc. 2004;126:3036–3037.Trend RM, Ramtohul YK, Stoltz BM. J Am Chem Soc. 2005;127:17778–17788.Yip KT, Yang M, Law KL, Zhu NY, Yang D. J Am Chem Soc. 2006;128:3130–3131.Zhang YJ, Wang F, Zhang W. J Org Chem. 2007;72:9208–9213.Tsujihara T, Shinohara T, Takenaka K, Takizawa S, Onitsuka K, Hatanaka M, Sasai H. J Org Chem. 2009;74:9274–9279.He W, Yip KT, Zhu NY, Yang D. Org Lett. 2009;11:5626–5628.Scarborough CC, Bergant A, Sazama GT, Guzei IA, Spencer LC, Stahl SS. Tetrahedron. 2009;65:5084–5092.Jiang F, Wu Z, Zhang W. Tetrahedron Lett. 2010;51:5124–5126.Mai DN, Wolfe JP. J Am Chem Soc. 2010;132:12157–12159.Jensen KH, Webb JD, Sigman MS. J Am Chem Soc. 2010;132:17471–17482.McDonald RI, White PB, Weinstein AB, Tam CP, Stahl SS. Org Lett. 2011;13:2830–2833.Yang G, Shen C, Zhang W. Angew Chem Int Ed. 2012;51:9141–9145.Angew Chem. 2012;124:9275–9279.
-
-
-
For examples of cis-nucleopalladation, see the following leading references: 4f,g,k,n and Ney JE, Wolfe JP. Angew Chem Int Ed. 2004;43:3605–3608.Angew Chem. 2004;116:3689–3692.Hay MB, Wolfe JP. J Am Chem Soc. 2005;127:16468–16476.Nakhla JS, Kampf JW, Wolfe JP. J Am Chem Soc. 2006;128:2893–2901.Liu G, Stahl SS. J Am Chem Soc. 2007;129:6328–6335.Muñiz K, Hovelmann CH, Streuff J. J Am Chem Soc. 2008;130:763–773.Michel BW, Steffens LD, Sigman MS. J Am Chem Soc. 2011;133:8317–8325.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

