The significant other: splicing by the minor spliceosome
- PMID: 23074130
- PMCID: PMC3584512
- DOI: 10.1002/wrna.1141
The significant other: splicing by the minor spliceosome
Abstract
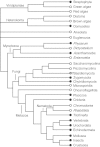
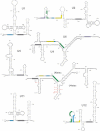
The removal of non-coding sequences, introns, from the mRNA precursors is an essential step in eukaryotic gene expression. U12-type introns are a minor subgroup of introns, distinct from the major or U2-type introns. U12-type introns are present in most eukaryotes but only account for less than 0.5% of all introns in any given genome. They are processed by a specific U12-dependent spliceosome, which is similar to, but distinct from, the major spliceosome. U12-type introns are spliced somewhat less efficiently than the major introns, and it is believed that this limits the expression of the genes containing such introns. Recent findings on the role of U12-dependent splicing in development and human disease have shown that it can also affect multiple cellular processes not directly related to the functions of the host genes of U12-type introns. At the same time, advances in understanding the regulation and phylogenetic distribution of the minor spliceosome are starting to shed light on how the U12-type introns and the minor spliceosome may have evolved.
Copyright © 2012 John Wiley & Sons, Ltd.
Figures




References
-
- Hall SL, Padgett RA. Conserved sequences in a class of rare eukaryotic nuclear introns with non-consensus splice-sites. J Mol Biol. 1994;239:357–365. - PubMed
-
- Burge CB, Padgett RA, Sharp PA. Evolutionary fates and origins of U12-type introns. Mol Cell. 1998;2:773–785. - PubMed
-
- Dietrich RC, Incorvaia R, Padgett RA. Terminal intron dinucleotide sequences do not distinguish between U2- and U12-dependent introns. Mol Cell. 1997;1:151–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

