The Hedgehog signal transduction network
- PMID: 23074268
- PMCID: PMC3705708
- DOI: 10.1126/scisignal.2002906
The Hedgehog signal transduction network
Abstract
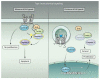
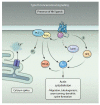
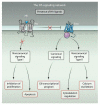
Hedgehog (Hh) proteins regulate the development of a wide range of metazoan embryonic and adult structures, and disruption of Hh signaling pathways results in various human diseases. Here, we provide a comprehensive review of the signaling pathways regulated by Hh, consolidating data from a diverse array of organisms in a variety of scientific disciplines. Similar to the elucidation of many other signaling pathways, our knowledge of Hh signaling developed in a sequential manner centered on its earliest discoveries. Thus, our knowledge of Hh signaling has for the most part focused on elucidating the mechanism by which Hh regulates the Gli family of transcription factors, the so-called "canonical" Hh signaling pathway. However, in the past few years, numerous studies have shown that Hh proteins can also signal through Gli-independent mechanisms collectively referred to as "noncanonical" signaling pathways. Noncanonical Hh signaling is itself subdivided into two distinct signaling modules: (i) those not requiring Smoothened (Smo) and (ii) those downstream of Smo that do not require Gli transcription factors. Thus, Hh signaling is now proposed to occur through a variety of distinct context-dependent signaling modules that have the ability to crosstalk with one another to form an interacting, dynamic Hh signaling network.
Figures

References
-
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15:3059–3087. - PubMed
-
- Hui CC, Angers S. Gli proteins in development and disease. Annu. Rev. Cell Dev. Biol. 2011;27:513–537. - PubMed
-
- Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. - PubMed
-
- Ruiz i Altaba A. Hedgehog signaling and the Gli code in stem cells, cancer, and metastases. Sci. Signal. 2011;4:pt9. - PubMed
-
- Bale AE. Hedgehog signaling and human disease. Annu. Rev. Genomics Hum. Genet. 2002;3:47–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

