CaMKII γ, a critical regulator of CML stem/progenitor cells, is a target of the natural product berbamine
- PMID: 23074277
- PMCID: PMC4507036
- DOI: 10.1182/blood-2012-06-434894
CaMKII γ, a critical regulator of CML stem/progenitor cells, is a target of the natural product berbamine
Abstract
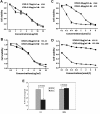
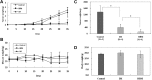
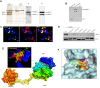
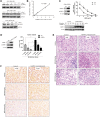
Bcr-Abl tyrosine kinase inhibitors (TKIs) have been a remarkable success for the treatment of Ph(+) chronic myeloid leukemia (CML). However, a significant proportion of patients treated with TKIs develop resistance because of leukemia stem cells (LSCs) and T315I mutant Bcr-Abl. Here we describe the unknown activity of the natural product berbamine that efficiently eradicates LSCs and T315I mutant Bcr-Abl clones. Unexpectedly, we identify CaMKII γ as a specific and critical target of berbamine for its antileukemia activity. Berbamine specifically binds to the ATP-binding pocket of CaMKII γ, inhibits its phosphorylation and triggers apoptosis of leukemia cells. More importantly, CaMKII γ is highly activated in LSCs but not in normal hematopoietic stem cells and coactivates LSC-related β-catenin and Stat3 signaling networks. The identification of CaMKII γ as a specific target of berbamine and as a critical molecular switch regulating multiple LSC-related signaling pathways can explain the unique antileukemia activity of berbamine. These findings also suggest that berbamine may be the first ATP-competitive inhibitor of CaMKII γ, and potentially, can serve as a new type of molecular targeted agent through inhibition of the CaMKII γ activity for treatment of leukemia.
Figures


References
-
- Hantschel O, Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol. 2004;5(1):33–44. - PubMed
-
- Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243(5405):290–293. - PubMed
-
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96(10):3343–3356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

