Cell sources for the regeneration of articular cartilage: the past, the horizon and the future
- PMID: 23075006
- PMCID: PMC3521894
- DOI: 10.1111/j.1365-2613.2012.00837.x
Cell sources for the regeneration of articular cartilage: the past, the horizon and the future
Abstract
Avascular, aneural articular cartilage has a low capacity for self-repair and as a consequence is highly susceptible to degradative diseases such as osteoarthritis. Thus the development of cell-based therapies that repair focal defects in otherwise healthy articular cartilage is an important research target, aiming both to delay the onset of degradative diseases and to decrease the need for joint replacement surgery. This review will discuss the cell sources which are currently being investigated for the generation of chondrogenic cells. Autologous chondrocyte implantation using chondrocytes expanded ex vivo was the first chondrogenic cellular therapy to be used clinically. However, limitations in expansion potential have led to the investigation of adult mesenchymal stem cells as an alternative cell source and these therapies are beginning to enter clinical trials. The chondrogenic potential of human embryonic stem cells will also be discussed as a developmentally relevant cell source, which has the potential to generate chondrocytes with phenotype closer to that of articular cartilage. The clinical application of these chondrogenic cells is much further away as protocols and tissue engineering strategies require additional optimization. The efficacy of these cell types in the regeneration of articular cartilage tissue that is capable of withstanding biomechanical loading will be evaluated according to the developing regulatory framework to determine the most appropriate cellular therapy for adoption across an expanding patient population.
© 2012 The Authors. International Journal of Experimental Pathology © 2012 International Journal of Experimental Pathology.
Figures
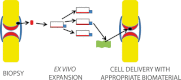
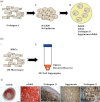
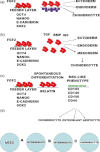

References
-
- Paget J. The classics. II. Healing of cartilage. Clin. Orthop. Relat. Res. 1969;64:7–8. - PubMed
-
- Azarin SM, Palecek SP. Matrix revolutions: a trinity of defined substrates for long-term expansion of human ESCs. Cell Stem Cell. 2010;7:7–8. - PubMed
-
- Bai HY, Chen GA, Mao GH, Song TR, Wang YX. Three step derivation of cartilage like tissue from human embryonic stem cells by 2D-3D sequential culture in vitro and further implantation in vivo on alginate/PLGA scaffolds. J. Biomed. Mater. Res. A. 2010;94:539–546. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

