Autophagy in periodontitis patients and gingival fibroblasts: unraveling the link between chronic diseases and inflammation
- PMID: 23075094
- PMCID: PMC3523085
- DOI: 10.1186/1741-7015-10-122
Autophagy in periodontitis patients and gingival fibroblasts: unraveling the link between chronic diseases and inflammation
Abstract
Background: Periodontitis, the most prevalent chronic inflammatory disease, has been related to cardiovascular diseases. Autophagy provides a mechanism for the turnover of cellular organelles and proteins through a lysosome-dependent degradation pathway. The aim of this research was to study the role of autophagy in peripheral blood mononuclear cells from patients with periodontitis and gingival fibroblasts treated with a lipopolysaccharide of Porphyromonas gingivalis. Autophagy-dependent mechanisms have been proposed in the pathogenesis of inflammatory disorders and in other diseases related to periodontitis, such as cardiovascular disease and diabetes. Thus it is important to study the role of autophagy in the pathophysiology of periodontitis.
Methods: Peripheral blood mononuclear cells from patients with periodontitis (n = 38) and without periodontitis (n = 20) were used to study autophagy. To investigate the mechanism of autophagy, we evaluated the influence of a lipopolysaccharide from P. gingivalis in human gingival fibroblasts, and autophagy was monitored morphologically and biochemically. Autophagosomes were observed by immunofluorescence and electron microscopy.
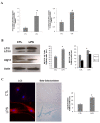
Results: We found increased levels of autophagy gene expression and high levels of mitochondrial reactive oxygen species production in peripheral blood mononuclear cells from patients with periodontitis compared with controls. A significantly positive correlation between both was observed. In human gingival fibroblasts treated with lipopolysaccharide from P. gingivalis, there was an increase of protein and transcript of autophagy-related protein 12 (ATG12) and microtubule-associated protein 1 light chain 3 alpha LC3. A reduction of mitochondrial reactive oxygen species induced a decrease in autophagy whereas inhibition of autophagy in infected cells increased apoptosis, showing the protective role of autophagy.
Conclusion: Results from the present study suggest that autophagy is an important and shared mechanism in other conditions related to inflammation or alterations of the immune system, such as periodontitis.
Figures




References
-
- World Health Organization. The world health report 2003 - shaping the future. Geneva: Geneva: World Health Organization; 2003.
-
- Kotseva K, Wood D, De Backer G, De Bacquer D, Pyörälä K, Keil U. EUROASPIRE Study Group. EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil. 2009;16:121–137. doi: 10.1097/HJR.0b013e3283294b1d. - DOI - PubMed
-
- Friedewald VE, Kornman KS, Beck JD, Genco R, Goldfine A, Libby P, Offenbacher S, Ridker PM, van Dyke TE, Roberts WC. American Journal of Cardiology; Journal of Periodontology. The American Journal of Cardiology and Journal of Periodontology Editors' Consensus: periodontitis and atherosclerotic cardiovascular disease. Am J Cardiol. 2009;104:59–68. doi: 10.1016/j.amjcard.2009.05.002. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

