Linezolid for treatment of chronic extensively drug-resistant tuberculosis
- PMID: 23075177
- PMCID: PMC3814175
- DOI: 10.1056/NEJMoa1201964
Linezolid for treatment of chronic extensively drug-resistant tuberculosis
Abstract
Background: Linezolid has antimycobacterial activity in vitro and is increasingly used for patients with highly drug-resistant tuberculosis.
Methods: We enrolled 41 patients who had sputum-culture-positive extensively drug-resistant (XDR) tuberculosis and who had not had a response to any available chemotherapeutic option during the previous 6 months. Patients were randomly assigned to linezolid therapy that started immediately or after 2 months, at a dose of 600 mg per day, without a change in their background regimen. The primary end point was the time to sputum-culture conversion on solid medium, with data censored 4 months after study entry. After confirmed sputum-smear conversion or 4 months (whichever came first), patients underwent a second randomization to continued linezolid therapy at a dose of 600 mg per day or 300 mg per day for at least an additional 18 months, with careful toxicity monitoring.
Results: By 4 months, 15 of the 19 patients (79%) in the immediate-start group and 7 of the 20 (35%) in the delayed-start group had culture conversion (P=0.001). Most patients (34 of 39 [87%]) had a negative sputum culture within 6 months after linezolid had been added to their drug regimen. Of the 38 patients with exposure to linezolid, 31 (82%) had clinically significant adverse events that were possibly or probably related to linezolid, including 3 patients who discontinued therapy. Patients who received 300 mg per day after the second randomization had fewer adverse events than those who continued taking 600 mg per day. Thirteen patients completed therapy and have not had a relapse. Four cases of acquired resistance to linezolid have been observed.
Conclusions: Linezolid is effective at achieving culture conversion among patients with treatment-refractory XDR pulmonary tuberculosis, but patients must be monitored carefully for adverse events. (Funded by the National Institute of Allergy and Infectious Diseases and the Ministry of Health and Welfare, South Korea; ClinicalTrials.gov number, NCT00727844.).
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



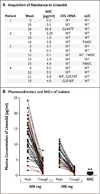
Comment in
-
Linezolid for extensively drug-resistant tuberculosis.N Engl J Med. 2013 Jan 17;368(3):290. doi: 10.1056/NEJMc1214183. N Engl J Med. 2013. PMID: 23323917 No abstract available.
-
Linezolid for extensively drug-resistant tuberculosis.N Engl J Med. 2013 Jan 17;368(3):290-1. doi: 10.1056/NEJMc1214183. N Engl J Med. 2013. PMID: 23323918 No abstract available.
-
Linezolid for extensively drug-resistant tuberculosis.N Engl J Med. 2013 Jan 17;368(3):291. doi: 10.1056/NEJMc1214183. N Engl J Med. 2013. PMID: 23323919 No abstract available.
-
Linezolid to treat extensively drug-resistant TB: retrospective data are confirmed by experimental evidence.Eur Respir J. 2013 Jul;42(1):288-90. doi: 10.1183/09031936.00191712. Eur Respir J. 2013. PMID: 23813314 No abstract available.
References
-
- Leach KL, Brickner SJ, Noe MC, Miller PF. Linezolid, the first oxazolidinone antibacterial agent. Ann N Y Acad Sci. 2011;1222:49–54. - PubMed
-
- Ippolito JA, Kanyo ZF, Wang D, et al. Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J Med Chem. 2008;51:3353–3356. - PubMed
-
- Vinh DC, Rubinstein E. Linezolid: a review of safety and tolerability. J Infect. 2009;59(Suppl 1):S59–S74. - PubMed
-
- Di Paolo A, Malacarne P, Guidotti E, Danesi R, Del Tacca M. Pharmacological issues of linezolid: an updated critical review. Clin Pharmacokinet. 2010;49:439–447. - PubMed
-
- Lee E, Burger S, Shah J, et al. Linezolid-associated toxic optic neuropathy: a report of 2 cases. Clin Infect Dis. 2003;37:1389–1391. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
