Impact of electrospun conduit fiber diameter and enclosing pouch pore size on vascular constructs grown within rat peritoneal cavities
- PMID: 23075322
- PMCID: PMC3589886
- DOI: 10.1089/ten.TEA.2012.0309
Impact of electrospun conduit fiber diameter and enclosing pouch pore size on vascular constructs grown within rat peritoneal cavities
Abstract
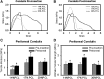
The generation of vascular grafts by recruiting autologous cells within the peritoneal cavity has shown promise. However, the microenvironment affects cell differentiation and elastic matrix production. Therefore, this study determined the impact of systematic changes in the average fiber diameter of electrospun poly(ɛ-caprolactone) conduits, and the pore size of pouches used to enclose the conduits, on recruited cells. After 2 weeks in the peritoneal cavity, fibrous capsules formed containing macrophages, α-smooth muscle actin (α-SMA)(+) and SM22α(+) myofibroblastic or smooth muscle like-cells, and what appeared to be mesothelial cells on the outer surfaces. These cells infiltrated and deposited matrix (e.g., collagen, hyaluoronan, and limited elastin) within conduit walls. Constructs enclosed within the largest pore pouches exhibited significantly better tissue generation responses (e.g., better cell infiltration, elongation, and matrix deposition). Additionally, the healing response was impacted by the conduit average fiber diameter, and consequently, the effective pore diameter, with the largest diameter fibers promoting the most positive healing response (e.g., greater total cellularity, extracellular matrix deposition, and α-SMA(+) cells). Six weeks post-intra-aortal grafting, constructs were occluded, but significant remodeling also occurred in the arterial microenvironment. Overall, these results demonstrate the importance of microenvironmental cues on recruited peritoneal cells and the necessity of developing strategies to further improve elastic matrix synthesis.
Figures






References
-
- McGill H.C., Jr. McMahan C.A. Herderick E.E. Malcom G.T. Tracy R.E. Strong J.P. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr. 2000;72:1307S. - PubMed
-
- Lloyd-Jones D. Adams R. Carnethon M. De Simone G. Ferguson T.B. Flegal K. Ford E. Furie K. Go A. Greenlund K. Haase N. Hailpern S. Ho M. Howard V. Kissela B. Kittner S. Lackland D. Lisabeth L. Marelli A. McDermott M. Meigs J. Mozaffarian D. Nichol G. O'Donnell C. Roger V. Rosamond W. Sacco R. Sorlie P. Stafford R. Steinberger J. Thom T. Wasserthiel-Smoller S. Wong N. Wylie-Rosett J. Hong Y. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21. - PubMed
-
- Klinkert P. Post P.N. Breslau P.J. van Bockel J.H. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg. 2004;27:357. - PubMed
-
- Chlupac J. Filova E. Bacakova L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol Res. 2009;58(Suppl 2):S119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

