Recent advances in the structural mechanisms of DNA glycosylases
- PMID: 23076011
- PMCID: PMC3530658
- DOI: 10.1016/j.bbapap.2012.10.005
Recent advances in the structural mechanisms of DNA glycosylases
Abstract
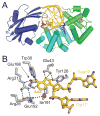
DNA glycosylases safeguard the genome by locating and excising a diverse array of aberrant nucleobases created from oxidation, alkylation, and deamination of DNA. Since the discovery 28years ago that these enzymes employ a base flipping mechanism to trap their substrates, six different protein architectures have been identified to perform the same basic task. Work over the past several years has unraveled details for how the various DNA glycosylases survey DNA, detect damage within the duplex, select for the correct modification, and catalyze base excision. Here, we provide a broad overview of these latest advances in glycosylase mechanisms gleaned from structural enzymology, highlighting features common to all glycosylases as well as key differences that define their particular substrate specificities.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
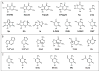
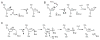
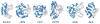
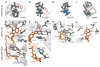
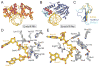

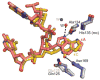

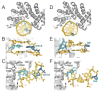

References
-
- Friedberg EC, Aguilera A, Gellert M, Hanawalt PC, Hays JB, Lehmann AR, Lindahl T, Lowndes N, Sarasin A, Wood RD. DNA repair: from molecular mechanism to human disease. DNA Repair (Amst) 2006;5:986–996. - PubMed
-
- Lindahl T. Suppression of spontaneous mutagenesis in human cells by DNA base excision-repair. Mutation research. 2000;462:129–135. - PubMed
-
- Dalhus B, Laerdahl JK, Backe PH, Bjørås M. DNA base repair--recognition and initiation of catalysis. FEMS Microbiol Rev. 2009;33:1044–1078. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

