Progression of chronic liver inflammation and fibrosis driven by activation of c-JUN signaling in Sirt6 mutant mice
- PMID: 23076146
- PMCID: PMC3516737
- DOI: 10.1074/jbc.M112.415182
Progression of chronic liver inflammation and fibrosis driven by activation of c-JUN signaling in Sirt6 mutant mice
Abstract
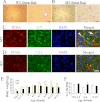
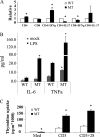
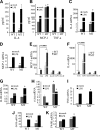
The human body has a remarkable ability to regulate inflammation, a biophysical response triggered by virus infection and tissue damage. Sirt6 is critical for metabolism and lifespan; however, its role in inflammation is unknown. Here we show that Sirt6-null (Sirt6(-/-)) mice developed chronic liver inflammation starting at ∼2 months of age, and all animals were affected by 7-8 months of age. Deletion of Sirt6 in T cells or myeloid-derived cells was sufficient to induce liver inflammation and fibrosis, albeit to a lesser degree than that in the global Sirt6(-/-) mice, suggesting that Sirt6 deficiency in the immune cells is the cause. Consistently, macrophages derived from the bone marrow of Sirt6(-/-) mice showed increased MCP-1, IL-6, and TNFα expression levels and were hypersensitive to LPS stimulation. Mechanistically, SIRT6 interacts with c-JUN and deacetylates histone H3 lysine 9 (H3K9) at the promoter of proinflammatory genes whose expression involves the c-JUN signaling pathway. Sirt6-deficient macrophages displayed hyperacetylation of H3K9 and increased occupancy of c-JUN in the promoter of these genes, leading to their elevated expression. These data suggest that Sirt6 plays an anti-inflammatory role in mice by inhibiting c-JUN-dependent expression of proinflammatory genes.
Figures


References
-
- Schonthaler H. B., Guinea-Viniegra J., Wagner E. F. (2011) Targeting inflammation by modulating the Jun/AP-1 pathway. Ann. Rheum. Dis. 70, (Suppl. 1) i109–i112 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

