S100A6 amyloid fibril formation is calcium-modulated and enhances superoxide dismutase-1 (SOD1) aggregation
- PMID: 23076148
- PMCID: PMC3516767
- DOI: 10.1074/jbc.M112.396416
S100A6 amyloid fibril formation is calcium-modulated and enhances superoxide dismutase-1 (SOD1) aggregation
Abstract
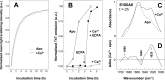
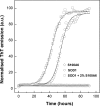
S100A6 is a small EF-hand calcium- and zinc-binding protein involved in the regulation of cell proliferation and cytoskeletal dynamics. It is overexpressed in neurodegenerative disorders and a proposed marker for Amyotrophic Lateral Sclerosis (ALS). Following recent reports of amyloid formation by S100 proteins, we investigated the aggregation properties of S100A6. Computational analysis using aggregation predictors Waltz and Zyggregator revealed increased propensity within S100A6 helices H(I) and H(IV). Subsequent analysis of Thioflavin-T binding kinetics under acidic conditions elicited a very fast process with no lag phase and extensive formation of aggregates and stacked fibrils as observed by electron microscopy. Ca(2+) exerted an inhibitory effect on the aggregation kinetics, which could be reverted upon chelation. An FT-IR investigation of the early conformational changes occurring under these conditions showed that Ca(2+) promotes anti-parallel β-sheet conformations that repress fibrillation. At pH 7, Ca(2+) rendered the fibril formation kinetics slower: time-resolved imaging showed that fibril formation is highly suppressed, with aggregates forming instead. In the absence of metals an extensive network of fibrils is formed. S100A6 oligomers, but not fibrils, were found to be cytotoxic, decreasing cell viability by up to 40%. This effect was not observed when the aggregates were formed in the presence of Ca(2+). Interestingly, native S1006 seeds SOD1 aggregation, shortening its nucleation process. This suggests a cross-talk between these two proteins involved in ALS. Overall, these results put forward novel roles for S100 proteins, whose metal-modulated aggregation propensity may be a key aspect in their physiology and function.
Figures





Similar articles
-
S100A6 and Its Brain Ligands in Neurodegenerative Disorders.Int J Mol Sci. 2020 Jun 1;21(11):3979. doi: 10.3390/ijms21113979. Int J Mol Sci. 2020. PMID: 32492924 Free PMC article. Review.
-
Calcium ions promote superoxide dismutase 1 (SOD1) aggregation into non-fibrillar amyloid: a link to toxic effects of calcium overload in amyotrophic lateral sclerosis (ALS)?J Biol Chem. 2013 Aug 30;288(35):25219-25228. doi: 10.1074/jbc.M113.470740. Epub 2013 Jul 16. J Biol Chem. 2013. PMID: 23861388 Free PMC article.
-
Calcium binding to gatekeeper residues flanking aggregation-prone segments underlies non-fibrillar amyloid traits in superoxide dismutase 1 (SOD1).Biochim Biophys Acta. 2015 Feb;1854(2):118-26. doi: 10.1016/j.bbapap.2014.11.005. Epub 2014 Nov 25. Biochim Biophys Acta. 2015. PMID: 25463043
-
S100A6, a calcium- and zinc-binding protein, is overexpressed in SOD1 mutant mice, a model for amyotrophic lateral sclerosis.Biochim Biophys Acta. 2000 Dec 20;1498(2-3):264-72. doi: 10.1016/s0167-4889(00)00101-4. Biochim Biophys Acta. 2000. PMID: 11108968
-
The Role of Metal Binding in the Amyotrophic Lateral Sclerosis-Related Aggregation of Copper-Zinc Superoxide Dismutase.Molecules. 2017 Aug 29;22(9):1429. doi: 10.3390/molecules22091429. Molecules. 2017. PMID: 28850080 Free PMC article. Review.
Cited by
-
Copper-induced structural propensities of the amyloidogenic region of human prion protein.J Biol Inorg Chem. 2014 Jun;19(4-5):635-45. doi: 10.1007/s00775-014-1132-7. Epub 2014 Apr 16. J Biol Inorg Chem. 2014. PMID: 24737041
-
Disassembly of Amyloid Fibril with Infrared Free Electron Laser.Int J Mol Sci. 2023 Feb 12;24(4):3686. doi: 10.3390/ijms24043686. Int J Mol Sci. 2023. PMID: 36835098 Free PMC article. Review.
-
Mutations in the vesicular trafficking protein annexin A11 are associated with amyotrophic lateral sclerosis.Sci Transl Med. 2017 May 3;9(388):eaad9157. doi: 10.1126/scitranslmed.aad9157. Sci Transl Med. 2017. PMID: 28469040 Free PMC article.
-
S100A6 and Its Brain Ligands in Neurodegenerative Disorders.Int J Mol Sci. 2020 Jun 1;21(11):3979. doi: 10.3390/ijms21113979. Int J Mol Sci. 2020. PMID: 32492924 Free PMC article. Review.
-
EFhd2 is a novel amyloid protein associated with pathological tau in Alzheimer's disease.J Neurochem. 2013 Jun;125(6):921-31. doi: 10.1111/jnc.12155. Epub 2013 Feb 14. J Neurochem. 2013. PMID: 23331044 Free PMC article.
References
-
- Fritz G., Heizmann C. W. (2004) in Handbook of Metalloproteins (Messerschmidt A., Huber R., Poulos T., Wieghardt K., eds), John Wiley & Sons
-
- Fritz G., Botelho H. M., Morozova-Roche L. A., Gomes C. M. (2010) Natural and amyloid self-assembly of S100 proteins: structural basis of functional diversity. FEBS J. 277, 4578–4590 - PubMed
-
- Botelho H. M., Koch M., Fritz G., Gomes C. M. (2009) Metal ions modulate the folding and stability of the tumor suppressor protein S100A2. Febs J. 276, 1776–1786 - PubMed
-
- Zimmer D. B., Wright Sadosky P., Weber D. J. (2003) Molecular mechanisms of S100-target protein interactions. Microsc. Res. Tech. 60, 552–559 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

