PAR1b takes the stage in the morphogenetic and motogenetic activity of Helicobacter pylori CagA oncoprotein
- PMID: 23076215
- PMCID: PMC3544774
- DOI: 10.4161/cam.21936
PAR1b takes the stage in the morphogenetic and motogenetic activity of Helicobacter pylori CagA oncoprotein
Abstract
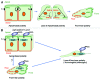
Helicobacter pylori CagA oncoprotein is critically involved in gastric carcinogenesis. Upon delivery into gastric epithelial cells via type IV secretion, CagA induces an extremely elongated cell-shape known as the hummingbird phenotype, which is associated with massive changes in actin cytoskeleton and elevated motility. With the notion that the hummingbird phenotype reflects pathogenic/oncogenic activity of CagA, many studies have focused on the mechanism through which CagA induces the morphological change. Once delivered, CagA interacts with host proteins such as oncogenic phosphatase SHP2 and polarity-regulating kinase PAR1b. Whereas the essential role of the CagA-SHP2 interaction in inducing the hummingbird phenotype has been extensively investigated, involvement of the CagA-PAR1b interaction in the morphological change has remained uncertain. Recently, we found that the CagA-PAR1b interaction, which inhibits PAR1b kinase activity, influences the actin cytoskeletal system and potentiates the magnitude of the hummingbird phenotype. We also found that PAR1b inactivates a RhoA-specific GEF, GEF-H1, via phosphorylation and thereby inhibits cortical actin and stress fiber formation. Collectively, these findings indicate that CagA-mediated inhibition of PAR1b promotes RhoA-dependent actin-cytoskeletal rearrangement and thereby strengthens the hummingbird phenotype induced by CagA-stimulated SHP2 during infection with H. pylori cagA-positive strains.
Figures


Comment on
-
Polarity-regulating kinase partitioning-defective 1b (PAR1b) phosphorylates guanine nucleotide exchange factor H1 (GEF-H1) to regulate RhoA-dependent actin cytoskeletal reorganization.J Biol Chem. 2011 Dec 30;286(52):44576-84. doi: 10.1074/jbc.M111.267021. Epub 2011 Nov 9. J Biol Chem. 2011. PMID: 22072711 Free PMC article.
-
Helicobacter pylori stimulates epithelial cell migration via CagA-mediated perturbation of host cell signaling.Microbes Infect. 2012 May;14(5):470-6. doi: 10.1016/j.micinf.2011.12.003. Epub 2011 Dec 11. Microbes Infect. 2012. PMID: 22202178
Similar articles
-
Natural variant of the Helicobacter pylori CagA oncoprotein that lost the ability to interact with PAR1.Cancer Sci. 2014 Mar;105(3):245-51. doi: 10.1111/cas.12342. Epub 2014 Feb 12. Cancer Sci. 2014. PMID: 24354359 Free PMC article.
-
Structural and functional diversity in the PAR1b/MARK2-binding region of Helicobacter pylori CagA.Cancer Sci. 2008 Oct;99(10):2004-11. doi: 10.1111/j.1349-7006.2008.00950.x. Cancer Sci. 2008. PMID: 19016760 Free PMC article.
-
Role of partitioning-defective 1/microtubule affinity-regulating kinases in the morphogenetic activity of Helicobacter pylori CagA.J Biol Chem. 2009 Aug 21;284(34):23024-36. doi: 10.1074/jbc.M109.001008. Epub 2009 Jun 24. J Biol Chem. 2009. PMID: 19553659 Free PMC article.
-
Sequence Polymorphism and Intrinsic Structural Disorder as Related to Pathobiological Performance of the Helicobacter pylori CagA Oncoprotein.Toxins (Basel). 2017 Apr 13;9(4):136. doi: 10.3390/toxins9040136. Toxins (Basel). 2017. PMID: 28406453 Free PMC article. Review.
-
Helicobacter pylori and gastric carcinogenesis.J Gastroenterol. 2009;44(4):239-48. doi: 10.1007/s00535-009-0014-1. Epub 2009 Mar 7. J Gastroenterol. 2009. PMID: 19271114 Review.
Cited by
-
Morphological changes in human gastric epithelial cells induced by nuclear targeting of Helicobacter pylori urease subunit A.J Microbiol. 2015 Jun;53(6):406-14. doi: 10.1007/s12275-015-5085-5. Epub 2015 May 30. J Microbiol. 2015. PMID: 26025173
-
Emerging Mechanisms and Treatment Progress on Liver Metastasis of Colorectal Cancer.Onco Targets Ther. 2021 May 6;14:3013-3036. doi: 10.2147/OTT.S301371. eCollection 2021. Onco Targets Ther. 2021. PMID: 33986602 Free PMC article. Review.
-
Role of Bacteria in the Incidence of Common GIT Cancers: The Dialectical Role of Integrated Bacterial DNA in Human Carcinogenesis.Infect Drug Resist. 2021 Jun 1;14:2003-2014. doi: 10.2147/IDR.S309051. eCollection 2021. Infect Drug Resist. 2021. PMID: 34103947 Free PMC article. Review.
-
CagA of Helicobacter pylori interacts with and inhibits the serine-threonine kinase PRK2.Cell Microbiol. 2015 Nov;17(11):1670-82. doi: 10.1111/cmi.12464. Epub 2015 Jun 19. Cell Microbiol. 2015. PMID: 26041307 Free PMC article.
-
Priming the seed: Helicobacter pylori alters epithelial cell invasiveness in early gastric carcinogenesis.World J Gastrointest Oncol. 2018 Sep 15;10(9):231-243. doi: 10.4251/wjgo.v10.i9.231. World J Gastrointest Oncol. 2018. PMID: 30254719 Free PMC article. Review.
References
-
- Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–9. - PubMed
-
- Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Jr., Chyou PH, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–5. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
