Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease
- PMID: 23076807
- PMCID: PMC3498515
- DOI: 10.1007/s00281-012-0351-7
Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease
Abstract
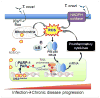
Chagas disease caused by Trypanosoma cruzi remains an important neglected tropical disease and a cause of significant morbidity and mortality. No longer confined to endemic areas of Latin America, it is now found in non-endemic areas due to immigration. The parasite may persist in any tissue, but in recent years, there has been increased recognition of adipose tissue both as an early target of infection and a reservoir of chronic infection. The major complications of this disease are cardiomyopathy and megasyndromes involving the gastrointestinal tract. The pathogenesis of Chagas disease is complex and multifactorial involving many interactive pathways. The significance of innate immunity, including the contributions of cytokines, chemokines, reactive oxygen species, and oxidative stress, has been emphasized. The role of the components of the eicosanoid pathway such as thromboxane A(2) and the lipoxins has been demonstrated to have profound effects as both pro- and anti-inflammatory factors. Additionally, we discuss the vasoconstrictive actions of thromboxane A(2) and endothelin-1 in Chagas disease. Human immunity to T. cruzi infection and its role in pathogen control and disease progression have not been fully investigated. However, recently, it was demonstrated that a reduction in the anti-inflammatory cytokine IL-10 was associated with clinically significant chronic chagasic cardiomyopathy.
Figures



References
-
- Araujo A, Jansen AM, Reinhard K, Ferreira LF. Paleoparasitology of Chagas disease--a review. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):9–16. - PubMed
-
- Cantey PT, Stramer SL, Townsend RL, Kamel H, Ofafa K, et al. The United States Trypanosoma cruzi Infection Study: evidence for vector-borne transmission of the parasite that causes Chagas disease among United States blood donors. Transfusion. 2012;52:1922–1930. - PubMed
-
- Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis. 2009;49:e52–54. - PubMed
-
- Basile L, Jansa JM, Carlier Y, Salamanca DD, Angheben A, et al. Chagas disease in European countries: the challenge of a surveillance system. Euro Surveill. 2011;16 - PubMed
Publication types
MeSH terms
Grants and funding
- HL73732/HL/NHLBI NIH HHS/United States
- HL094802/HL/NHLBI NIH HHS/United States
- AI076248/AI/NIAID NIH HHS/United States
- HL088230/HL/NHLBI NIH HHS/United States
- R21 HL088230/HL/NHLBI NIH HHS/United States
- T32 AI070117/AI/NIAID NIH HHS/United States
- R01 AI076248/AI/NIAID NIH HHS/United States
- AI054578/AI/NIAID NIH HHS/United States
- R01 HL073732/HL/NHLBI NIH HHS/United States
- R21 AI068538/AI/NIAID NIH HHS/United States
- R01 HL094802/HL/NHLBI NIH HHS/United States
- AI06538/AI/NIAID NIH HHS/United States
- R01 AI054578/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

