Duloxetine versus other anti-depressive agents for depression
- PMID: 23076926
- PMCID: PMC4169791
- DOI: 10.1002/14651858.CD006533.pub2
Duloxetine versus other anti-depressive agents for depression
Abstract
Background: Although pharmacological and psychological interventions are both effective for major depression, in primary and secondary care settings antidepressant drugs remain the mainstay of treatment. Amongst antidepressants many different agents are available. Duloxetine hydrochloride is a dual reuptake inhibitor of serotonin and norepinephrine and has been licensed by the Food and Drug Administration in the US for major depressive disorder (MDD), generalised anxiety disorder, diabetic peripheral neuropathic pain, fibromyalgia and chronic musculoskeletal pain.
Objectives: To assess the evidence for the efficacy, acceptability and tolerability of duloxetine in comparison with all other antidepressant agents in the acute-phase treatment of major depression.
Search methods: MEDLINE (1966 to 2012), EMBASE (1974 to 2012), the Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register and the Cochrane Central Register of Controlled Trials up to March 2012. No language restriction was applied. Reference lists of relevant papers and previous systematic reviews were hand-searched. Pharmaceutical company marketing duloxetine and experts in this field were contacted for supplemental data.
Selection criteria: Randomised controlled trials allocating patients with major depression to duloxetine versus any other antidepressive agent.
Data collection and analysis: Two review authors independently extracted data and a double-entry procedure was employed. Information extracted included study characteristics, participant characteristics, intervention details and outcome measures in terms of efficacy, acceptability and tolerability.
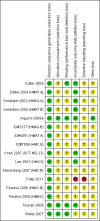
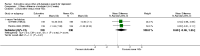
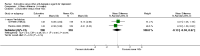
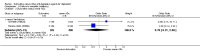
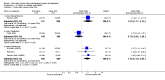
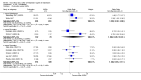
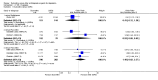
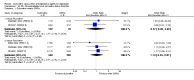
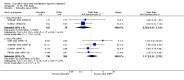
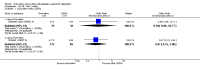
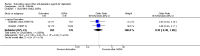
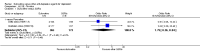
Main results: A total of 16 randomised controlled trials (overall 5735 participants) were included in this systematic review. Of these, three trials were unpublished. We found 11 studies (overall 3304 participants) comparing duloxetine with one selective serotonin reuptake inhibitor (SSRI) (six studies versus paroxetine, three studies versus escitalopram and two versus fluoxetine), four studies (overall 1978 participants) comparing duloxetine with a newer antidepressants (three with venlafaxine and one with desvenlafaxine, respectively) and one study (overall 453 participants) comparing duloxetine with an antipsychotic drug which is also used as an antidepressive agent, quetiapine. No studies were found comparing duloxetine with tricyclic antidepressants. The pooled confidence intervals were rather wide and there were no statistically significant differences in efficacy when comparing duloxetine with other antidepressants. However, when compared with escitalopram or venlafaxine, there was a higher rate of drop out due to any cause in the patients randomised to duloxetine (odds ratio (OR) 1.62; 95% confidence interval (CI) 1.01 to 2.62 and OR 1.56; 95% CI 1.14 to 2.15, respectively). There was also some weak evidence suggesting that patients taking duloxetine experienced more adverse events than paroxetine (OR 1.24; 95% CI 0.99 to 1.55).
Authors' conclusions: Duloxetine did not seem to provide a significant advantage in efficacy over other antidepressive agents for the acute-phase treatment of major depression. No differences in terms of efficacy were found, even though duloxetine was worse than some SSRIs (most of all, escitalopram) and newer antidepressants (like venlafaxine) in terms of acceptability and tolerability. Unfortunately, we only found evidence comparing duloxetine with a handful of other active antidepressive agents and only a few trials per comparison were found (in some cases we retrieved just one trial). This limited the power of the review to detect moderate, but clinically meaningful differences between the drugs. As many statistical tests have been used in the review, the findings from this review are better thought of as hypothesis forming rather than hypothesis testing and it would be very comforting to see the conclusions replicated in future trials. Most of included studies were sponsored by the drug industry manufacturing duloxetine. As for all other new investigational compounds, the potential for overestimation of treatment effect due to sponsorship bias should be borne in mind. In the present review no trials reported economic outcomes. Given that several SSRIs and the great majority of antidepressants are now available as generic formulation (only escitalopram, desvenlafaxine and duloxetine are still on patent), more comprehensive economic estimates of antidepressant treatment effect should be considered to better inform healthcare policy.
Conflict of interest statement
AC, CB, MN, IMO, CT, MP, SD, MK: none declared.
TAF has received honoraria for speaking at CME meetings sponsored by Asahi Kasei, Eli Lilly, GlaxoSmithKline, Mochida, MSD, Otsuka, Pfizer, Shionogi and Tanabe‐Mitsubishi. He is diplomate of the Academy of Cognitive Therapy. He has received royalties from Igaku‐Shoin, Seiwa‐Shoten and Nihon Bunka Kagaku‐sha. He is on advisory board for Sekisui Chemicals and Takeda Science Foundation. The Japanese Ministry of Education, Science, and Technology, the Japanese Ministry of Health, Labor and Welfare, and the Japan Foundation for Neuroscience and Mental Health have funded his research projects.
Figures




































































































Update of
References
References to studies included in this review
Cutler 2009 {published data only}
-
- Cutler AJ, Montgomery SA, Feifel D, Lazarus A, Astrom M, Brecher M. Extended release quetiapine fumarate monotherapy in major depressive disorder: a placebo‐ and duloxetine‐controlled study. Journal of Clinical Psychiatry 2009;70(4):526‐39. - PubMed
Detke 2004 (HMAY A) {published data only}
-
- Detke MJ, Wiltse CG, Mallinckrodt CH, McNamara RK, Demitrack MA, Bitter I. Duloxetine in the acute and long‐term treatment of major depressive disorder: a placebo‐ and paroxetine‐controlled trial. Europen Neuropsychopharmacology 2004;14(6):457‐70. - PubMed
-
- Eli Lilly Trial. Duloxetine versus placebo and paroxetine in the treatment of major depression [Trial 4298a, HMAYAa]. www.clinicalstudyresults.org/documents/company‐study_887_0.pdf [accessed 5 October 2007, archived data on file].
Goldstein 2002 (HMAQ A) {published and unpublished data}
-
- Demitrack MA, Goldstein DJ, Mallinckrodt C, Lu Y. Efficacy and safety of duloxetine treatment in major depression. 154th Annual Meeting of American Psychiatric Association. 2001 May 5‐10; New Orleans.
-
- Eli Lilly. Duloxetine versus placebo in the treatment of major depression [Trial 3327a, HAMAQ A]. http://www.clinicalstudyresults.org/documents/company‐study_137_0.pdf [accessed 5 October 2007, archived data on file].
-
- Goldstein DJ, Mallinckrodt C, Lu Y, Demitrack MA. Duloxetine in the treatment of major depressive disorder: a double‐blind clinical trial. Journal Clinical Psychiatry 2002;63(3):225‐31. - PubMed
Goldstein 2004 (HMAT B) {published and unpublished data}
-
- Eli Lilly. Duloxetine versus placebo and paroxetine in the acute treatment of major depression [Trial 4091b, HMAT B]. http://www.clinicalstudyresults.org/documents/company‐study_139_0.pdf [accessed 5 October 2007, archived data on file].
-
- Goldstein DJ, Lu Y, Dekte MJ, Wiltse CG, Mallinckrodt C, Demitrack MA. Duloxetine versus paroxetine in the treatment of depression. 155th Annual Meeting of American Psychiatric Association. 2002 May 18‐23; Philadelphia.
-
- Goldstein DJ, Lu Y, Detke M, Wiltse C, Mallinckrodt C, Demitrack MA. Duloxetine in the treatment of depression: a double blind placebo controlled comparison with paroxetine. European Neuropsychopharmacology 2002;12:43‐4. - PubMed
-
- Goldstein DJ, Lu Y, Detke MJ, Wiltse C, Mallinckrodt C, Demitrack MA. Duloxetine in the treatment of depression: a double‐blind placebo‐controlled comparison with paroxetine. Journal Clinical Psychopharmacology 2004;24(4):389‐99. - PubMed
-
- Mallinckrodt C, Goldstein DJ, Lu Y, Detke M, Wiltse C, Demitrack MA. Duloxetine in the treatment of depression: a double blind placebo controlled comparison with Paroxetine. European Neuropsychopharmacology 2002;12(Suppl 3):S214. - PubMed
Higuchi 2009a {published data only}
-
- Higuchi T, Murasaki M, Kamijima K. Clinical evaluation of duloxetine in the treatment of major depressive disorder placebo and paroxetine controlled double‐blind comparative study. Japanese Journal of Clinical Psychopharmacology 2009;12:1613‐34.
ID#3327 (HMAQ B) {unpublished data only}
-
- Eli Lilly. Duloxetine versus placebo in the treatment of major depression [Trial 3327b, HMAQ B]. http://www.clinicalstudyresults.org/documents/company‐study_138_0.pdf [accessed 5 October 2007, archived data on file].
ID#4091 (HMAT A) {unpublished data only}
-
- Eli Lilly. Duloxetine versus placebo and paroxetine in the acute treatment of major depression, Study Group A [Trial 4091a, HMAT A]. http://www.clinicalstudyresults.org/documents/company‐study_170_0.pdf [accessed 5 October 2007, archived data on file].
ID#7999 (HMCQ) {unpublished data only}
-
- Eli Lilly. Duloxetine versus venlafaxine extended release in the treatment of major depressive disorder [Trial 7999, HMCQ]. http://www.clinicalstudyresults.org/documents/company‐study_3108_0.pdf [accessed 1 December 2010, archived data on file].
Khan 2007 (SCT‐MD‐23) {published data only}
-
- Forest Laboratories. Double‐blind study of escitalopram in adult patients with major depressive disorder [Trial SCT‐MD‐23]. Forest Laboratories Clinical Trial Registry [http://www.forestclinicaltrials.com/] 2007.
-
- Khan A, Bose A, Alexopoulos GS, Gommoll C, Li D, Gandhi C. Double‐blind comparison of escitalopram and duloxetine in the acute treatment of major depressive disorder. Clinical Drug Investigation 2007;27(7):481‐92. - PubMed
Lee 2007 (HMCV) {published data only}
-
- Eli Lilly. Duloxetine versus paroxetine in the acute treatment of major depression [Trial 6937]. http://www.clinicalstudyresults.org/documents/company‐study_2402_0.pdf [accessed 5 October 2007, archived data on file].
-
- Lee P, Shu L, Xu X, Wang CY, Lee MS, Liu CY, et al. Once‐daily duloxetine 60 mg in the treatment of major depressive disorder: multicenter, double‐blind, randomized, paroxetine‐controlled, non‐inferiority trial in China, Korea, Taiwan and Brazil. Psychiatry and Clinical Neurosciences 2007;61(3):295‐307. - PubMed
Nierenberg 2007 (HMCR) {published data only}
-
- Eli Lilly. Duloxetine versus escitalopram and placebo in the treatment of patients with major depression [Trial 7978, HMCR]. http://www.clinicalstudyresults.org/documents/company‐study_2182_0.pdf [accessed 5 October 2007, archived data on file].
-
- Nierenberg AA, Greist JH, Mallinckrodt CH, Prakash A, Sambunaris A, Tollefson GD, et al. Duloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non‐inferiority study. Current Medical Research and Opinion 2007;23(2):401‐16. - PubMed
-
- Nierenberg AA, Griest JH, Mallinckrodt CH, Prakash A, Watkin JG, Sambunaris A, et al. Onset of antidepressant action and acute efficacy and safety of duloxetine versus escitalopram and placebo in the treatment of major depressive disorder. Neuropsychopharmacology 2005;30(Suppl 1):S142.
-
- Pigott TA, Prakash A, Arnold LM, Aaronson ST, Mallinckrodt CH, Wohlreich MM. Duloxetine versus escitalopram and placebo: an 8‐month, double‐blind trial in patients with major depressive disorder. Current Medical Research and Opinion 2007;23(6):1303‐18.;23(6):1303‐18. - PubMed
Patel 2011 {published data only}
-
- Badyal DK, Khosla PP, Deswal RS, Matreja PS. Safety and efficacy of duloxetine versus venlafaxine in major depression in Indian patients. JK Science 2006;8(4):195‐9.
-
- Patel DM, Mehta HR, Shah SK, Srivastava AA. An open label randomized, comparative, parallel group, multicentric clinical trial to evaluate the safety and efficacy of duloxetine compared to venlafaxine in patients with major depression. Pharma Science Monitor: An International Journal of Pharmaceutical Sciences 2011;2(2):126‐40.
Perahia 2006 (HMAY B) {published data only}
-
- Eli Lilly. Duloxetine versus placebo and paroxetine in the treatment of major depression [ Trial 4298b, HMAYb]. http://www.clinicalstudyresults.org/documents/company‐study_1618_0.pdf [accessed 5 October 2007, archived data on file].
-
- Perahia DG, Wang F, Mallinckrodt CH, Walker DJ, Detke MJ. Duloxetine in the treatment of major depressive disorder: a placebo‐ and paroxetine‐controlled trial. European Psychiatry 2006;21(6):367‐78. - PubMed
Perahia 2008 (HMBU) {published and unpublished data}
-
- Eli Lilly. Duloxetine versus venlafaxine extended release in the treatment of major depressive disorder [Trial 6090, HMBU]. http://www.clinicalstudyresults.org/documents/company‐study_3107_0.pdf [accessed 19 August 2010, archived data on file].
-
- Perahia DG, Pritchett YL, Kajdasz DK, Bauer M, Jain R, Russell JM, et al. A randomized, double‐blind comparison of duloxetine and venlafaxine in the treatment of patients with major depressive disorder. Journal of Psychiatric Research 2008;42(1):22‐34. - PubMed
Tourian 2009 {published and unpublished data}
-
- Pfizer. A multicenter, randomized, double‐blind, placebo‐controlled, duloxetine‐referenced, parallel‐group study to evaluate the efficacy and safety of 2 fixed doses (50mg, 100mg) of desvenlafaxine sustained‐release tablets in adult outpatients with major depressive disorder [results]. ClinicalTrials.gov 2012. http://www.clinicaltrials.gov/ct2/show/results/NCT00384033.
-
- Tourian KA, Padmanabhan SK, Groark J, Brisard C, Farrington D. Desvenlafaxine 50 and 100 mg/d in the treatment of major depressive disorder: an 8‐week, phase III, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group trial and a post hoc pooled analysis of three studies. Clinical Therapeutics 2009;31(1):1405‐23. - PubMed
-
- Wyeth. Final report: a multicenter, randomized, double‐blind, placebo‐controlled, duloxetine‐referenced, parallel‐group study to evaluate the efficacy and safety of 2 fixed doses (50 mg, 100 mg) of desvenlafaxine sustained release tablets in adult outpatients with major depressive disorder. Synopsis based on final report. (Protocol 3151A1‐335) [NCT00384033]. Clinicalstudyresults.org [www.clinicalstudyresults.org] 2008 [accessed 28 March 2011, archived data on file].
Wade 2007 {published data only}
-
- H Lundbeck A/S. A double‐blind, randomised, multicenter, comparative study of escitalopram and duloxetine in outpatients with major depressive disorder [Trial 10990]. http://www.lundbecktrials.com/Data/PDFs/10990_CTRS_Final%20for%20upload_....
-
- Wade A, Gembert K, Florea I. A comparative study of the efficacy of acute and continuation treatment with escitalopram versus duloxetine in patients with major depressive disorder. Current Medical Research and Opinion 2007;23(7):1605‐14. - PubMed
-
- Wade AG, Fernandez J‐L, Francois C, Hansen K, Danchenko N, Despiegel N. Escitalopram and duloxetine in major depressive disorder: A pharmacoeconomic comparison using UK cost data. PharmacoEconomics 2008;26(11):969‐81. - PubMed
References to studies excluded from this review
Badyal 2006 {published data only}
-
- Badyal DK, Khosla PP, Deswal RS, Matreja PS. Safety and efficacy of duloxetine versus venlafaxine in major depression in Indian patients. K Science 2006;8(4):195‐9.
Herrera‐Guzman 2009 {published data only}
-
- Herrera‐Guzman I, Gudayol‐Ferre E, Herrera‐Guzman D, Guardia‐Olmos J, Hinojosa‐Calvo E, Herrera‐Abarca JE. Effects of selective serotonin reuptake and dual serotonergic‐noradrenergic reuptake treatments on memory and mental processing speed in patients with major depressive disorder. Journal of Psychiatric Research 2009;43(9):855‐63. - PubMed
Higuchi 2009b {published data only}
-
- Higuchi T, Murasaki M, Kamijima. Clinical evaluation of duloxetine in the treatment of major depressive disorder: Superiority of study of 40 mg and 60 mg versus 5 mg. Rinsho Seishin Yakuri 2009;12:1595‐612.
ID#1126 (HMAI) {unpublished data only}
-
- Brunton S, Wang F, Edwards SB, Crucitti AS, Ossanna MJ, Walker DJ, et al. Profile of adverse events with duloxetine treatment: a pooled analysis of placebo‐controlled studies. Drug Safety: an international journal of medical toxicology and drug experience 2010;33(5):393‐407. - PubMed
-
- Eli Lilly. A double‐blind, placebo‐ and clomipramine‐controlled study in duloxetine patients with major depression [Trial 1126, HMAI]. http://www.clinicalstudyresults.org/documents/company‐study_4148_0.pdf [accessed 1 December 2010, archived data on file].
Lam 2008 {published data only}
-
- Lam RW, Andersen HF, Wade AG. Escitalopram and duloxetine in the treatment of major depressive disorder: a pooled analysis of two trials. International Clinical Psychopharmacology 2008;23(4):181‐7. - PubMed
Lam 2010 {published data only}
-
- Lam RW, Lonn SL, Despiegel N. Escitalopram versus serotonin noradrenaline reuptake inhibitors as second step treatment for patients with major depressive disorder: A pooled analysis. International Clinical Psychopharmacology 2010;25(4):199‐203. - PubMed
Murasaki 2009a {published data only}
-
- Murasaki M. Clinical evaluation of duloxetine in the treatment of depression and depressive state: Mianserin‐controlled double‐blind comparative study. Rinsho Seishin Yakuri 2009;12:1533‐48.
Murasaki 2009b {published data only}
-
- Murasaki M. Clinical evaluation of duloxetine in the treatment of depression and depressive state: Imipramine‐controlled double‐blind comparative study. Rinsho Seishin Yakuri 2009;12:1517‐31.
NCT00666757 {unpublished data only}
-
- Eli Lilly, Boehringer Ingelheim Pharmaceuticals. TRY FIRST: A 12‐week, randomized, open‐label trial of duloxetine versus generic SSRIs in the treatment of a severe depressive episode [NCT00666757]. ClinicalTrials.gov 2008. http://clinicaltrials.gov/ct2/show/NCT00666757.
-
- Martinez JM, Katon W, Greist JH, Kroenke K, Thase ME, Meyers AL, et al. A pragmatic 12‐week, randomized trial of duloxetine versus generic selective serotonin‐reuptake inhibitors in the treatment of adult outpatients in a moderate‐to‐severe depressive episode. International Clinical Psychopharmacology 2012;27(1):17‐26. - PubMed
NCT00810068 {unpublished data only}
-
- Eli Lilly. Comparison of two different treatment strategies in patients with major depressive disorder not exhibiting improvement on escitalopram treatment: early vs. delayed intervention strategy [NCT00810068; F1J‐EW‐HMGD; EU 2008‐002319‐42]. ClinicalTrials.gov 2008. http://clinicaltrials.gov/ct2/show/NCT00810069.
Perahia 2008 {published data only}
-
- Perahia DG, Pritchett YL, Kajdasz DK, Bauer M, Jain R, Russell JM. A randomized, double‐blind comparison of duloxetine and venlafaxine in the treatment of patients with major depressive disorder. Journal of Psychiatric Research 2008;42(1):22‐34. - PubMed
Romera 2010 {published data only}
-
- Romera I, Perez V, Menchon JM, Schacht A, Papen R, Neuhauser D. Early vs. delayed switch to duloxetine in patients with major depressive disorder not exhibiting early improvement with escitalopram: A double‐blind randomized study. International Journal of Psychiatry in Clinical Practice 2010;14:34.
SCT‐MD‐39 {unpublished data only}
-
- Asnis G, Tsai J, Mao Y. Fixed‐dose comparison of escitalopram and duloxetine in severely depressed patients. Poster presented at Collegium Internationale Neuro‐Psychopharmacologicum XXVI; July 15, 2008; Munich, Germany. 2008.
-
- Forest Laboratories. Fixed dose comparison of escitalopram to an active comparator in severely depressed patients [Trial SCT‐MD‐39]. Forset Laboratories Clinical Trial Registry [www.forestclinicaltrials.com] 2006.
Serafini 2010 {published data only}
-
- Serafini G, Pompili M, Casale A, Mancini M, Innamorati M, Lester D. Duloxetine versus venlafaxine in the treatment of unipolar and bipolar depression. Clinica Terapeutica 2010;161(4):321‐7. - PubMed
Tsutsui 2009 {published data only}
-
- Tsutsui S. Clinical evaluation of duloxetine in the treatment of depression and depressive state: Trazodone‐controlled double‐blind comparative study. Rinsho Seishin Yakuri 2009;12:1549‐63.
References to studies awaiting assessment
Joubert 1997 {published data only}
-
- Joubert AF, du Plessis AD, Faries D, Gagiano CA. High Placebo response rate versus clinical impression with the new antidepressant Duloxetine. Sith World Congress of Biological Psychiatry. 1997 June 22‐27; Nice.
NCT00635219 {unpublished data only}
-
- Baldwin D, Loft H, Dragheim M. A randomised, double‐blind, placebo controlled, duloxetine‐referenced, fixed‐dose study of three dosages of Lu AA21004 in MDD treatment [conference abstract]. European Neuropsychopharmacology [abstracts of the 24th Congress of the European College of Neuropsychopharmacology, ECNP; 2011 Sept 3‐7, Paris France] 2011;21((Suppl 3)):S390. - PubMed
-
- Baldwin DS, Loft H, Dragheim M. A randomised, double‐blind, placebo controlled,duloxetine‐referenced, fixed‐dose study of threedosages of Lu AA21004 in acute treatment of majordepressive disorder (MDD). European Neuropsychopharmacology. 2011:doi:10.1016/j.euroneuro.2011.11.008. - PubMed
-
- H Lundbeck A/S . A randomised, double‐blind, parallel‐group, placebo‐controlled, duloxetine‐referenced, fixed‐dose study evaluating the efficacy and safety of three dosages of Lu AA21004, in acute treatment of major depressive disorder [NCT00635219]. ClinicalTrials.gov 2008. http://www.clinicaltrials.gov/ct2/show/NCT00635219.
NCT00672620 {unpublished data only}
-
- H Lundbeck A/S. A randomized, double‐blind, parallel‐group, placebo‐controlled, active‐referenced, fixed‐dose study comparing the efficacy and safety of 2 doses of Lu AA21004 in acute treatment of adults with major depressive disorder [NCT00672620]. ClinicalTrials.gov 2008. http://www.clinicaltrials.gov/ct2/show/NCT00672620.
NCT00811252 {unpublished data only}
-
- H Lundbeck A/S. Randomised, double‐blind, parallel‐group, placebo‐controlled, duloxetine‐referenced, fixed dose study comparing the efficacy and safety of Lu AA21004 in acute treatment of major depressive disorder in elderly patients [NCT00811252]. ClinicalTrials.gov 2008. http://www.clinicaltrials.gov/ct2/show/NCT00811252.
-
- Katona C, Hansen T, Olsen CK. A randomized, double‐blind, placebo‐controlled, duloxetine‐referenced, fixed‐dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. International Clinical Psychopharmacology 2012;27(4):215‐23. - PubMed
NCT01145755 {unpublished data only}
-
- AstraZeneca. A phase IIa, multi‐centre, randomized, double‐blind, double‐dummy, active and placebo controlled, parallel group study to assess the effectiveness and safety of AZD2066 after 6 weeks of treatment in patients with major depressive disorder ‐ D0475C00020. [NCT01145755]. ClinicalTrials.gov 2010. http://clinicaltrials.gov/ct2/show/NCT01145755.
References to ongoing studies
2008‐004642‐92 {unpublished data only}
-
- Servier Laboratories. Evaluation of efficacy and clinical benefit of agomelatine (25 to 50 mg/day) over a 6‐month treatment period in patients with major depressive disorder.A randomised, double‐blind, international multicentre study with parallel groups versus duloxetine (60 mg/day).Twenty‐four weeks of treatment. [Servier Trial CL3‐20098‐062; EudraCT Number: 2008‐004642‐92]. EU Clinical Trials Register [www.clinicaltrialsregister.eu/ctr‐search/] 2009.
NCT01140906 {unpublished data only}
-
- H Lundbeck A/S. A randomised, double‐blind, parallel‐group, placebo‐controlled, duloxetine‐referenced, fixed‐dose study evaluating the efficacy and safety of Lu AA21004 (15 and 20 mg/Day) in the acute treatment of adult patients with major depressive disorder [NCT01140906]. ClinicalTrials.gov 2010. http://clinicaltrials.gov/ct2/show/NCT01140906 2010.
NCT01153009 {unpublished data only}
-
- Takeda. A phase 3, randomized, double‐blind, parallel‐group, placebo‐controlled, duloxetine‐referenced, fixed‐dose study comparing the efficacy and safety of 2 doses (15 and 20 mg) of Lu AA21004 in acute treatment of adults with major depressive disorder [NCT01153009]. ClinicalTrials.gov 2010 http://clinicaltrials.gov/ct2/show/NCT01153009 2010.
NCT01288079 {unpublished data only}
-
- AstraZeneca. A phase IIb, randomized, double‐blind, placebo‐controlled, active controlled, parallel group, multicenter study to assess the safety and efficacy of 2 fixed dose groups of TC‐5214 (S‐mecamylamine) as monotherapy treatment in patients with major depressive disorder who exhibit an inadequate response to antidepressant therapy [NCT01288079; D4131C00001; EU 2010‐023816‐15]. http://www.clinicaltrials.gov/ct2/show/NCT01288079.
Additional references
Als‐Nielsen 2003
-
- Als‐Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events?. JAMA 2003;290(7):921‐8. - PubMed
Altman 1996
Anderson 2000
-
- Anderson IM, Nutt DJ, Deakin JF. Evidence‐based guidelines for treating depressive disorders with antidepressants: a revision of the 1993 British Association for Psychopharmacology guidelines. Journal of Psychopharmacology 2000;14(1):3‐20. - PubMed
APA 1994
-
- American Psychiatric Association. .. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 4th Edition. Washington, DC: American Psychiatric Association, 1994.
APA 2000
-
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. American Journal of Psychiatry 2000;157(4 Suppl):1‐45. - PubMed
APA 2006
-
- American Psychiatric Association. American Psychiatric Association Practice Guidelines for the Treatment of Psychiatric Disorders: Compendium 2006. American Psychiatric Association, 2006.
Barbui 2004
-
- Barbui C, Cipriani A, Brambilla P, Hotopf M. "Wish bias" in antidepressant drug trials?. Journal of Clinical Psychopharmacology 2004;24(2):126‐30. - PubMed
Bhandari 2004
Bollini 1999
-
- Bollini P, Pampallona S, Tibaldi G, Kupelnick B, Munizza C. Effectiveness of antidepressants. Meta‐analysis of dose‐effect relationships in randomised clinical trials. British Journal of Psychiatry 1999;174:297‐303. - PubMed
Buchkowsky 2004
-
- Buchkowsky SS, Jewesson PJ. Industry sponsorship and authorship of clinical trials over 20 years. Annals of Pharmacotherapy 2004;38(4):579‐85. - PubMed
Bymaster 2001
-
- Bymaster FP, Dreshfield‐Ahmad LJ, Threlkeld PG, Shaw JL, ThompsonL, Nelson DL, et al. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology 2001;25:871‐80. - PubMed
Castren 2005
-
- Castrén E. Is mood chemistry?. Nature Reviews Neuroscience 2005;6(3):241‐6. - PubMed
Cipriani 2005
Cipriani 2006
-
- Cipriani A, Barbui C, Brambilla P, Furukawa TA, Hotopf M, Geddes JR. Are all antidepressants really the same? The case of fluoxetine: A systematic review. Journal of Clinical Psychiatry 2006;67(6):850‐64. - PubMed
Cipriani 2009a
-
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new‐generation antidepressants: a multiple‐treatments meta‐analysis. Lancet 2009;373:746–58. - PubMed
Cipriani 2009b
Cipriani 2009c
Cipriani 2009d
-
- Cipriani A, Purgato M, Barbui C. Why internal and external validity of experimental studies are relevant for clinical practice?. Epidemiology and Psychiatric Sciences 2009;18(2):101‐3. - PubMed
Cipriani 2009e
-
- Cipriani A, Geddes JR. What is a randomised controlled trial?. Epidemiologia e Psichiatria Sociale 2009;18(3):191‐4. - PubMed
Cipriani 2012
Ciuna 2004
-
- Ciuna A, Andretta M, Corbari L, Levi D, Mirandola M, Sorio A, et al. Are we going to increase the use of antidepressants up to that of benzodiazepines?. European Journal of Clinical Pharmacology 2004;60(9):629‐34. - PubMed
Depression Guideline Panel 1993
-
- Depression Guideline Panel. Depression in primary care: Vol 2. Treatment of Major Depression, Clinical Practice Guideline, Number 5, AHCPR Publication No. 93‐0551. Rockville, MD: U. S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, 1993.
Dozois 2004
-
- Dozois D J A, Dobson K S. Depression. In: Antony M M, Barlow D H editor(s). Handbook of Assessment and Treatment Planning for Psychological Disorders. New York: Guilford Press, 2004:259‐99.
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] - PubMed
Feighner 1972
-
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry 1972;26:57‐63. - PubMed
Frampton 2007
-
- Frampton JE, Plosker GL. Duloxetine: a review of its use in the treatment of major depressive disorder. CNS Drugs 2007;21(7):581‐609. - PubMed
Furukawa 2002a
-
- Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta‐analyses. International Journal of Epidemiology 2002;31(1):72‐6. - PubMed
Furukawa 2002b
Furukawa 2005
-
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta‐analysis. International Clinical Psychopharmacology 2005;20(1):49‐52. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. - PubMed
Furukawa 2007
-
- Furukawa T A, Watanabe N, Omori I M, Montori V M, Guyatt G H. Association between unreported outcomes and effect size estimates in Cochrane meta‐analyses. JAMA 2007;297(5):468‐70. - PubMed
Gartlehner 2009
-
- Gartlehner G, Thaler K, Hansen RA, Gaynes BN. The general and comparative efficacy and safety of duloxetine in major depressive disorder: a systematic review and meta‐analysis. Drug Safety 2009;32(12):1159‐73. - PubMed
Gartlehner 2010
Guy 1976
-
- Guy W. Clinical Global Impressions ‐ ECDEU Asessment Manual Psychopharmacology (DHEW Publ No ADM 76‐338). Revised. Rockville MD: U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH, 1976.
Guyatt 1998
Hall 1995
-
- Hall RC. Global Assessment of Functioning ‐ a modified scale. Psychosomatics 1995;36:267‐75. - PubMed
Hamilton 1960
Hansen 2005
-
- Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second‐generation antidepressants in the treatment of major depressive disorder. Annals of Internal Medicine 2005;143(6):415‐26. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].. The Cochrane Collaboration, 2011.. Available from www.cochrane‐handbook.org., 2011.
Ioannidis 2005
Khan 2003
-
- Khan A, Khan SR, Walens G, Kolts R, Giller EL. Frequency of positive studies among fixed and flexible dose antidepressant clinical trials: an analysis of the food and drug administration summary basis of approval reports. Neuropsychopharmacology 2003;28(3):552‐7. - PubMed
Lexchin 2003
Linde 2008
Maher 2011
-
- Maher AR, Maglione M, Bagley S, Suttorp M, Hu JH, Ewing B, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications for off‐label uses in adults: a systematic review and meta‐analysis. JAMA 2011;306(12):1359‐69. - PubMed
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. - PubMed
Montgomery 2004
-
- Montgomery JH, Byerly M, Carmody T, Li B, Miller DR, Varghese F, et al. An analysis of the effect of funding source in randomized clinical trials of second generation antipsychotics for the treatment of schizophrenia. Controlled Clinical Trials 2004;25(6):598‐612. - PubMed
Mottram 2006
Müller 2003
-
- Müller M J, Himmerich H, Kienzle B, Szegedi A. Differentiating moderate and severe depression using the Montgomery‐Asberg depression rating scale (MADRS). Journal of Affective Disorders 2003;77(3):255‐60. - PubMed
Nakagawa 2009
NICE 2010
-
- National Institute for Clinical Excellence. Depression: management of depression in primary and secondary care (http://guidance.nice.org.uk/CG90). London: National Institute for Clinical Excellence, 2010.
Omori 2010
Oxman 1992
-
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. - PubMed
Perlis 2005
-
- Perlis RH, Perlis CS, Wu Y, Hwang C, Joseph M, Nierenberg AA. Industry sponsorship and financial conflict of interest in the reporting of clinical trials in psychiatry. American Journal of Psychiatry 2005 Oct;162(10):1957‐60. - PubMed
Procyshyn 2004
-
- Procyshyn RM, Chau A, Fortin P, Jenkins W. Prevalence and outcomes of pharmaceutical industry‐sponsored clinical trials involving clozapine, risperidone, or olanzapine. Canadian Journal of Psychiatry 2004;49(9):601‐6. - PubMed
Rothwell 2005
-
- Rothwell PM. External validity of randomised controlled trials: to whom do the results apply?. Lancet 2005;365:82–93. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408–12. - PubMed
Spitzer 1972
-
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Archives General Psychiatry 1978;35(6):773‐82. - PubMed
Stahl 2000
-
- Stahl SM. Essential Psychopharmacology. 2nd Edition. Cambridge University Press, 2000.
Sterne 2000
-
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. Journal of clinical epidemiology 2000;53(11):1119‐29. [PUBMED: 11106885] - PubMed
Suh 1997
-
- Suh T, Gallo J J. Symptom profiles of depression among general medical service users compared with specialty mental health service users. Psychological Medicine 1997;27(5):1051‐63. - PubMed
Taylor 2006
Thase 2010
-
- Thase ME, Nierenberg AA, Vrijland P, Oers HJ, Schutte AJ, Simmons JH. Remission with mirtazapine and selective serotonin reuptake inhibitors: a meta‐analysis of individual patient data from 15 controlled trials of acute phase treatment of major depression. International Clinical Psychopharmacology 2010;25:189‐98. - PubMed
Turner 2008
-
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. New England Journal of Medicine 2008;358(3):252‐60. - PubMed
Ware 1992
-
- Ware JE, Sherbourne CD. The MOS 36‐item short form health survey (SF‐36). Medical Care 1992;30:473‐83. - PubMed
Ware 1998
-
- Ware JE, Kosinski M, Keller SD. SF‐12: How to Score the SF‐12.Physical and Mental Health Summary Scales. Lincoln RI: QualityMetric Inc, 1998.
Watanabe 2011
WHO 1992
-
- World Health Organization. The Tenth Revision of the International Classification of Diseases and Related Health Problems (ICD‐10). Geneva: World Health Organization, 1992.
WHO 2006
-
- World Health Organization. WHO Collaborative Centre for Drug Statistics Methodology. http://www.whocc.no/atcddd/.
WHO 2009a
-
- WHO Collaborative Centre for Drug Statistics Methodology. ATC/DDD Index 2009. http://www.whocc.no/atcddd/ 2009.
WHOQOL Group 1998
-
- WHOQOL Group. The World Health Organization quality of life assessment (WHOQOL): Development and general psychometric properties. Social Science and Medicine 1998;46(12):1569‐85. - PubMed
Wing 1998
-
- Wing JK, Beevor AS, Curtis RH, Park SBG, Burns A. Health of the nation outcome scales (HoNOS): Research and development. British Journal of Psychiatry 1998;172:11‐8. - PubMed
Wong 2002
-
- Wong DT, Bymaster FP. Dual serotonin and noradrenaline uptake inhibitor class of antidepressants potential for greater efficacy or just hype?. Progres in Drug Research 2002;58:169‐222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

