Postoperative tamoxifen for ductal carcinoma in situ
- PMID: 23076938
- PMCID: PMC11955262
- DOI: 10.1002/14651858.CD007847.pub2
Postoperative tamoxifen for ductal carcinoma in situ
Abstract
Background: Ductal carcinoma in situ (DCIS) is a non-invasive carcinoma of the breast. The incidence of DCIS has increased substantially over the last twenty years, largely as a result of the introduction of population-based mammographic screening. The treatment of DCIS tumours involves surgery with or without radiotherapy to prevent recurrent DCIS and invasive carcinoma. However, there is clinical uncertainty as to whether postoperative hormonal treatment (tamoxifen) after surgery confers benefit in overall survival and incidence of recurrent carcinoma.
Objectives: To assess the effects of postoperative tamoxifen in women having local surgical resection of DCIS.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library), the Cochrane Breast Cancer Group's Specialised Register, and the World Health Organization's International Clinical Trials Registry Platform (WHO ICTRP) on 16 August 2011.
Selection criteria: Published and unpublished randomised controlled trials (RCTs) and quasi-randomised controlled trials comparing tamoxifen after surgery for DCIS (regardless of oestrogen receptor status), with or without adjuvant radiotherapy.
Data collection and analysis: Two authors independently assessed trial quality and extracted data. Statistical analyses were performed using the fixed-effect model and the results were expressed as relative risks (RRs) or hazard ratios (HRs) with 95% confidence intervals (CIs).
Main results: We included two RCTs involving 3375 women. Tamoxifen after surgery for DCIS reduced recurrence of both ipsilateral (same side) DCIS (HR 0.75; 95% CI 0.61 to 0.92) and contralateral (opposite side) DCIS (RR 0.50; 95% CI 0.28 to 0.87). There was a trend towards decreased ipsilateral invasive cancer (HR 0.79; 95% CI 0.62 to 1.01) and reduced contralateral invasive cancer (RR 0.57; 95% CI 0.39 to 0.83). The number needed to treat in order for tamoxifen to have a protective effect against all breast events is 15. There was no evidence of a difference detected in all cause mortality (RR 1.11; 95% CI 0.89 to 1.39). Only one study, involving 1799 participants followed-up for 163 months (median) reported on adverse events (i.e. toxicity, mood changes, deep vein thrombosis, pulmonary embolism, endometrial cancer) with no significant difference between tamoxifen and placebo groups, but there was a non-significant trend towards more endometrial cancer in the tamoxifen group.
Authors' conclusions: While tamoxifen after local excision for DCIS (with or without adjuvant radiotherapy) reduced the risk of recurrent DCIS (in the ipsi- and contralateral breast), it did not reduce the risk of overall mortality.
Conflict of interest statement
No authors have any conflict of interest relating to this review.
Figures
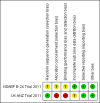
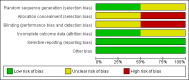

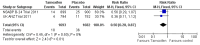

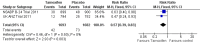





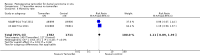







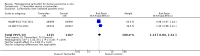
Comment in
-
CNCF podcast: postoperative tamoxifen in women with ductal carcinoma in situ.Int J Nurs Pract. 2014 Dec;20(6):685. doi: 10.1111/ijn.12384. Int J Nurs Pract. 2014. PMID: 25532882 No abstract available.
References
References to studies included in this review
NSABP B‐24 Trial 2011 {published data only}
-
- Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B‐24 randomised controlled trial. Lancet 1999;353(9169):1993‐2000. - PubMed
-
- Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Seminars in Oncology 2001;28(4):400‐18. - PubMed
-
- Wolmark N, Dignam J, Fisher B. The addition of tamoxifen to lumpectomy and radiotherapy in the treatment of ductal carcinoma in situ (DCIS) preliminary results of NSABP protocol B‐24. Breast Cancer Research & Treatment. 1998; Vol. 50 (3):227.
-
- Wolmark N, Dunn BK. The role of tamoxifen in breast cancer prevention: issues sparked by the NSABP Breast Cancer Prevention Trial (P‐1). Annals of the New York Academy of Sciences. 2001; Vol. 949:99‐108. - PubMed
UK ANZ Trial 2011 {published data only}
-
- George WD, Houghton J, Cuzick J, Forbes J. Radiotherapy and tamoxifen following complete local excision (CLE) in the management of ductal carcinoma in situ (DCIS): Preliminary results from the UK DCIS Trial. Proceedings of the American Society of Clinical Oncology. 2000.
-
- Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M, UK Coordinating Committee on Cancer Research, Ductal Carcinoma in situ Working Party, & DCIS trialists in the UK, ANZ. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet 2003;362(9378):95‐102. - PubMed
References to studies excluded from this review
Assersohn 1999 {published data only}
-
- Assersohn L, Powles TJ, Ashley S, Nash AG, Neal AJ, Sacks N, et al. Local relapse in primary breast cancer patients with unexcised positive surgical margins after lumpectomy, radiotherapy and chemoendocrine therapy. Annals of Oncology 1999;10(12):1451‐5. - PubMed
Conley 2000 {published data only}
-
- Conley B, O'Shaughnessy J, Prindiville S, Lawrence J, Chow C, Jones E, et al. Pilot trial of the safety, tolerability, and retinoid levels of N‐(4‐hydroxyphenyl) retinamide in combination with tamoxifen in patients at high risk for developing invasive breast cancer. Journal of Clinical Oncology 2000;18(2):275‐83. - PubMed
Cuzick 2002 {published data only}
-
- Cuzick J, Forbes J, Edwards R, Baum M, Cawthorn S, Coates A, et al. First results from the International Breast Cancer Intervention Study (IBIS‐I): a randomised prevention trial. Lancet 2002;360(9336):817‐24. - PubMed
Cuzick 2007 {published data only}
-
- Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, et al. Long‐term results of tamoxifen prophylaxis for breast cancer‐‐96‐month follow‐up of the randomized IBIS‐I trial. Journal of the National Cancer Institute 2007;99(4):272‐82. - PubMed
Cuzick 2009 {unpublished data only}
-
- Cuzick J. Adjuvant tamoxifen compared with anastrozole in treating postmenopausal women with ductal carcinoma in situ. ClinicalTrials.gov (http://clinicaltrials.gov).
Decensi 2009 {published data only}
Euhus (in progress) {unpublished data only}
-
- Euhus, D. Phase II randomized chemoprevention study of tamoxifen in women at increased risk for breast cancer. Physician Data Query / ClinicalTrial.gov website (registered in 2004).
Fabian 2004 {published data only}
-
- Fabian CJ, Kimler BF, Anderson J, Tawfik OW, Mayo MS, Burak WE Jr, et al. Breast cancer chemoprevention phase I evaluation of biomarker modulation by arzoxifene, a third generation selective estrogen receptor modulator. Clinical Cancer Research 2004;10(16):5403‐17. - PubMed
Guerrieri‐Gonzaga 2006 {published data only}
-
- Guerrieri‐Gonzaga A, Robertson C, Bonanni B, Serrano D, Cazzaniga M, Mora S, et al. Preliminary results on safety and activity of a randomized, double‐blind, 2 x 2 trial of low‐dose tamoxifen and fenretinide for breast cancer prevention in premenopausal women. Journal of Clinical Oncology 2006;24(1):129‐35. - PubMed
Hamilton 1990 {published data only}
Hamilton 1992 {published data only}
-
- Hamilton C. Ethical and practical problems in trials testing treatment for pre‐malignant conditions: breast cancer as a model. In: Williams CJ editor(s). Introducing New Treatments for Cancer: Practical, Ethical and Legal Problems. Wiley, 1992:315‐21.
Lee 2008 {published and unpublished data}
-
- Barrett LP. A UK randomised trial for the management of screen‐detected DCIS. Cochrane Breast Cancer Group Specialised Register.
Powles 2007 {published data only}
-
- Powles T, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty‐year follow‐up of the Royal Marsden randomized, double‐blinded tamoxifen breast cancer prevention trial. Journal of the National Cancer Institute 2007;99(4):283‐90. - PubMed
Whelan 2006 {unpublished data only}
-
- Radiation therapy with or without optimal tamoxifen in treating women with ductal carcinoma in situ. Physician Data Query Registered on ClinicalTrials.gov in 2006.
References to ongoing studies
Garcia {unpublished data only}
-
- Garcia A. Randomized pilot study of neoadjuvant fulvestrant versus tamoxifen in postmenopausal women with newly diagnosed ductal carcinoma in situ of the breast. Physician Data Query (PDQ) 2005.
Margolese {unpublished data only}
-
- Margolese RG. Phase III randomized study of anastrozole versus tamoxifen in post menopausal women with ductal carcinoma in situ of the breast undergoing lumpectomy and radiotherapy. Physician Data Query (PDQ) 2003.
Additional references
(BIG) 1‐98 2005
-
- Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. New England Journal of Medicine 2005;353:2747‐53. - PubMed
ATAC 2006
-
- Buzdar A, Howell A, Cuzick J, Wale C, Distler W, Hoctin‐Boes G, et al. Comprehensive side‐effect profile of anastrozole and tamoxifen as adjuvant treatment for early‐stage breast cancer: long‐term safety analysis of the ATAC trial. Lancet Oncology 2006;7:633‐43. - PubMed
Bijker 2001
EBCTCG 2001
-
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000486] - DOI
Fisher 1998
-
- Fisher D, Dignam J, Wolmark N, Mamounas E, Costantino J, Poller W. Lumpectomy and radiation therapy for treatment of intraductal breast cancer: findings from adjuvant surgical national breast & bowel project B17. Journal of Clinical Oncology 1998;16:441‐52. - PubMed
Goodsell 2002
-
- Goodsell DS. The molecular perspective: tamoxifen and the estrogen receptor. Oncologist 2002;7:163‐4. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org. The Cochrane Collaboration.
Julien 2000
-
- Julien JP, Bijker N, Fentiman IS, Peterse JL, Delledonne V, Rouanet P, et al. Radiotherapy in breast‐conserving treatment for ductal carcinoma in situ ‐ first result of the EORTC phase III randomised trial 10853. EORTC Breast cancer cooperative group & EORTC Radiotherapy group. Lancet 2000;355:528‐33. - PubMed
Kerlikowske 2003
-
- Kerlikowske K, Molinaro A, Cha I, Ljung BM, Ernster VL, Stewart K, et al. Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. Journal of the National Cancer Institute 2003;95:1692‐702. - PubMed
Page 1995
-
- Page DL, Dupont WD, Rogers LW, Jensen RA, Schuyler PA. Continued local recurrence of carcinoma 15‐25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer 1995;76:1197‐2000. - PubMed
Petrelli 2011
-
- Petrelli F, Barni S. Tamoxifen added to radiotherapy and surgery for the treatment of ductal carcinoma in situ of the breast: a meta‐analysis of 2 randomized trials. Radiotherapy and Oncology 2011;100(2):195‐9. - PubMed
RevMan [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Tierney 2007
van Steenbergen 2008
-
- Steenbergen LN, Voogd AC, Roukema JA, Louwman WJ, Duijm LE, Coebergh JW, et al. Screening caused rising incidence rates of ductal carcinoma in situ of the breast. Breast Cancer Research and Treatment 2008;115(1):181‐3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

