Venom immunotherapy for preventing allergic reactions to insect stings
- PMID: 23076950
- PMCID: PMC8734599
- DOI: 10.1002/14651858.CD008838.pub2
Venom immunotherapy for preventing allergic reactions to insect stings
Abstract
Background: Venom immunotherapy (VIT) is commonly used for preventing further allergic reactions to insect stings in people who have had a sting reaction. The efficacy and safety of this treatment has not previously been assessed by a high-quality systematic review.
Objectives: To assess the effects of immunotherapy using extracted insect venom for preventing further allergic reactions to insect stings in people who have had an allergic reaction to a sting.
Search methods: We searched the following databases up to February 2012: the Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library, MEDLINE (from 1946), EMBASE (from 1974), PsycINFO (from 1806), AMED (from 1985), LILACS (from 1982), the Armed Forces Pest Management Board Literature Retrieval System, and OpenGrey. There were no language or publication status restrictions to our searches. We searched trials databases, abstracts from recent European and North American allergy meetings, and the references of identified review articles in order to identify further relevant trials.
Selection criteria: Randomised controlled trials of venom immunotherapy using standardised venom extract in insect sting allergy.
Data collection and analysis: Two authors independently undertook study selection, data extraction, and assessment of risk of bias. We identified adverse events from included controlled trials and from a separate analysis of observational studies identified as part of a National Institute for Health and Clinical Excellence Health Technology Assessment.
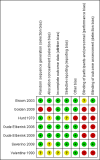
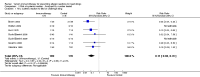
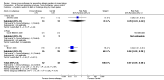
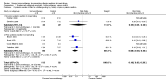
Main results: We identified 6 randomised controlled trials and 1 quasi-randomised controlled trial for inclusion in the review; the total number of participants was 392. The trials had some risk of bias because five of the trials did not blind outcome assessors to treatment allocation. The interventions included ant, bee, and wasp immunotherapy in children or adults with previous systemic or large local reactions to a sting, using sublingual (one trial) or subcutaneous (six trials) VIT. We found that VIT is effective for preventing systemic allergic reaction to an insect sting, which was our primary outcome measure. This applies whether the sting occurs accidentally or is given intentionally as part of a trial procedure.In the trials, 3/113 (2.7%) participants treated with VIT had a subsequent systemic allergic reaction to a sting, compared with 37/93 (39.8%) untreated participants (risk ratio [RR] 0.10, 95% confidence interval [CI] 0.03 to 0.28). The efficacy of VIT was similar across studies; we were unable to identify a patient group or mode of treatment with different efficacy, although these analyses were limited by small numbers. We were unable to confirm whether VIT prevents fatal reactions to insect stings, because of the rarity of this outcome.Venom immunotherapy was also effective for preventing large local reactions to a sting (5 studies; 112 follow-up stings; RR 0.41, 95% CI 0.24 to 0.69) and for improving quality of life (mean difference [MD] in favour of VIT 1.21 points on a 7-point scale, 95% CI 0.75 to 1.67).We found a significant risk of systemic adverse reaction to VIT treatment: 6 trials reported this outcome, in which 14 of 150 (9.3%) participants treated with VIT and 1 of 135 (0.7%) participants treated with placebo or no treatment suffered a systemic reaction to treatment (RR 8.16, 95% CI 1.53 to 43.46; 2 studies contributed to the effect estimate). Our analysis of 11 observational studies found systemic adverse reactions occurred in 131/921 (14.2%) participants treated with bee venom VIT and 8/289 (2.8%) treated with wasp venom VIT.
Authors' conclusions: We found venom immunotherapy using extracted insect venom to be an effective therapy for preventing further allergic reactions to insect stings, which can improve quality of life. The treatment carries a small but significant risk of systemic adverse reaction.
Conflict of interest statement
Robert Boyle has received research funding from Lincoln Medical Ltd and support for conference attendance from Meda Pharmaceuticals, both of which market adrenaline autoinjector devices, and his department has received research support from Allergy Therapeutics who market allergen immunotherapy products, including venom immunotherapy.
Hanneke Oude Elberink (J.N.G. Oude Elberink) undertook two of the trials included in this review. She has received research support from ALK‐Abello who market allergen immunotherapy, and HAL Allergy; she has received honorarium from MSD, Allergopharma, ALK‐Abello, and AstraZeneca; and she has received fees for consulting from ALK‐Abello and HAL Allergy.
Mariam Elremeli, Max Bulsara, Juliet Hockenhull, Gemma Cherry, and Michael Daniels have no interests to declare.
A clinical referee on the review, Dr Cristoforo Incorvaia, has received fees as a scientific consultant from the producer of allergen extracts, Stallergenes.
Figures













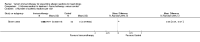














Update of
References
References to studies included in this review
Brown 2003 {published and unpublished data}
-
- Brown SG, Wiese MD, Blackman KE, Heddle RJ. Ant venom immunotherapy: a double‐blind, placebo‐controlled, crossover trial. Lancet 2003;361(9362):1001‐6. - PubMed
Golden 2009 {published and unpublished data}
-
- Golden DB, Kelly D, Hamilton RG, Craig TJ. Venom immunotherapy reduces large local reactions to insect stings. Journal of Allergy and Clinical Immunology 2009;123(6):1371‐5. - PubMed
Hunt 1978 {published and unpublished data}
-
- Hunt KJ, Valentine MD, Sobotka AK, Benton A, Amodio FJ, Lichtenstein LM. A controlled trial of immunotherapy in insect hypersensitivity. New England Journal of Medicine 1978;299(4):157‐61. - PubMed
Oude Elberink 2006 {published and unpublished data}
-
- Oude Elberink JNG, Monchy JGR, Guyatt GH, Dubois AEJ. Venom immunotherapy improves health‐related quality of life in patients with allergic reactions following yellow‐jacket stings ‐ extended observations. Journal of Allergy and Clinical Immunology 2001;107(2):S222‐3.
-
- Oude Elberink JNG, Monchy JGR, Heide S, Guyatt GH, Dubois AEJ. Venom immunotherapy improves health‐related quality of life in patients allergic to yellow jacket venom. Journal of Allergy and Clinical Immunology 2002;110(1):174‐82. - PubMed
-
- Oude Elberink JNG, Heide S, Guyatt GH, Dubois AEJ. Analysis of the burden of treatment in patients receiving an EpiPen for yellow jacket anaphylaxis. Journal of Allergy & Clinical Immunology 2006;118(3):699‐704. - PubMed
Oude Elberink 2009 {published and unpublished data}
-
- Oude Elberink JNG, Heide S, Guyatt GH, Dubois AEJ. Immunotherapy improves health‐related quality of life of adult patients with dermal reactions following yellow jacket stings. Clinical and Experimental Allergy 2009;39(6):883‐9. - PubMed
Severino 2008 {published and unpublished data}
-
- Severino M, Cortellini, G, Bonadonna P, Francescato E, Panzini I, Macchia D, et al. Sublingual immunotherapy with honeybee venom reduces large local reactions: a randomised, double blind controlled study. EAACI Conference Abstract. 2008:A170. - PubMed
-
- Severino MG, Cortellini G, Bonadonna P, Francescato E, Panzini I, Macchia D, et al. Sublingual immunotherapy for large local reactions caused by honeybee sting: a double‐blind, placebo‐controlled trial. Journal of Allergy and Clinical Immunology 2008;122(1):44‐8. - PubMed
Valentine 1990 {published data only (unpublished sought but not used)}
-
- Schuberth KC, Lichtenstein LM, Kagey‐Sobotka A, Szklo M, Kwiterovich KA, Valentine MD. Epidemiologic study of insect allergy in children. II. Effect of accidental stings in allergic children. Journal of Pediatrics 1983;102(3):361‐5. - PubMed
-
- Valentine MD, Schuberth KC, Kagey‐Sobotka A, Graft DF, Kwiterovich KA, Szklo M, et al. The value of immunotherapy with venom in children with allergy to insect stings. New England Journal of Medicine 1990;323(23):1601‐3. - PubMed
References to studies excluded from this review
Berchtold 1992 {published data only}
-
- Berchtold E, Maibach R, Müller U. Reduction of side effects from rush‐immunotherapy with honey bee venom by pretreatment with terfenadine. Clinical and Experimental Allergy 1992;22(1):59‐65. - PubMed
Bilo 2009 {published data only}
-
- Bilò MB, Severino M, Cilia M, Pio A, Casino G, Ferrarini E, et al. The VISYT trial: Venom Immunotherapy Safety and Tolerability with purified vs nonpurified extracts. Annals of Allergy Asthma and Immunology 2009;103(1):57‐61. - PubMed
Bousquet 1987 {published data only}
-
- Bousquet J, Fontez A, Aznar R, Robinet‐Levy M, Michel FB. Combination of passive and active immunization in honeybee venom immunotherapy. Journal of Allergy and Clinical Immunology 1987;79(6):947‐54. - PubMed
Brockow 1997 {published data only}
-
- Brockow K, Kiehn M, Riethmüller C, Vieluf D, Berger J, Ring J. Efficacy of antihistamine pretreatment in the prevention of adverse reactions to Hymenoptera immunotherapy: a prospective, randomized, placebo‐controlled trial. Journal of Allergy and Clinical Immunology 1997;100(4):458‐63. - PubMed
Brown 2008 {published data only}
-
- Brown SG, Wiese MD, Chuter CL, Gunner J. Rapid (ultra‐rush) versus clustered (semi rush) initiation of insect venom immunotherapy: an open randomised controlled trial with patient choice arms. Internal Medicine Journal 2008;38(S6):A151.
Divanovic 1997 {published data only}
-
- Divanovic A, Melac M, Herman D. Cetirizine (20 mg/day) versus placebo in the prevention of reactions secondary to accelerated desensitization to hymenoptera venoms [Cetirizine (20 mg/j) versus placebo dans la prevention des reactions secondaires de la desensibilisation acceleree aux venins d'hymenopteres]. Revue Francaise D'Allergologie Et D'Immunologie Clinique 1997;37(6):741‐5.
Malling 1985 {published data only}
-
- Malling HJ, Djurup R, Søndergaard I, Weeke B. Clustered immunotherapy with yellow jacket venom. Evaluation of the influence of time interval on in vivo and in vitro parameters. Allergy 1985;40(5):373‐83. - PubMed
Mosbech 1986 {published data only}
-
- Mosbech H, Malling H J, Biering I, Böwadt H, Søborg M, Weeke B, et al. Immunotherapy with yellow jacket venom. A comparative study including three different extracts, one adsorbed to aluminium hydroxide and two unmodified. Allergy 1986;41(2):95‐103. - PubMed
Muller 1985 {published data only}
-
- Müller U, Lanner A, Schmid P, Bischof M, Dreborg S, Hoigné R. A double blind study on immunotherapy with chemically modified honey bee venom: monomethoxy polyethylene glycol‐coupled versus crude honey bee venom. International Archives of Allergy and Applied Immunology 1985;77(1‐2):201‐3. - PubMed
Muller 1987 {published data only}
-
- Müller U, Rabson AR, Bischof M, Lomnitzer R, Dreborg S, Lanner A. A double‐blind study comparing monomethoxy polyethylene glycol‐modified honeybee venom and unmodified honeybee venom for immunotherapy. Journal of Allergy and Clinical Immunology 1987;80(3 Pt 1):252‐61. - PubMed
Muller 1992 {published data only}
-
- Müller U, Helbling A, Berchtold E. Immunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safety. Journal of Allergy and Clinical Immunology 1992;89(2):529‐35. - PubMed
Muller 2001 {published data only}
-
- Müller U, Hari Y, Berchtold E. Premedication with antihistamines may enhance efficacy of specific‐allergen immunotherapy. Journal of Allergy and Clinical Immunology 2001;107(1):81‐6. - PubMed
Muller 2008 {published data only}
-
- Müller UR, Jutel M, Reimers A, Zumkehr J, Huber C, Kriegel C, et al. Clinical and immunologic effects of H1 antihistamine preventive medication during honeybee venom immunotherapy. Journal of Allergy and Clinical Immunology 2008;122(5):1001‐7. - PubMed
Ohman 1986 {published data only}
-
- Öhman S, Björkander J, Dreborg S, Lanner Å, Malling HJ, Weeke B. A preliminary study of immunotherapy with a monomethoxy polyethylene glycol modified honey bee venom preparation. Allergy 1986;41(2):81‐8. - PubMed
Quercia 2001 {published data only}
-
- Quercia O, Rafanelli S, Puccinelli P, Stefanini GF. The safety of cluster immunotherapy with aluminium hydroxide‐adsorbed honey bee venom extract. Journal of Investigative Allergology and Clinical Immunology 2001;11(1):27‐33. - PubMed
Reimers 2000 {published data only}
-
- Reimers A, Hari Y, Müller U. Reduction of side‐effects from ultrarush immunotherapy with honeybee venom by pretreatment with fexofenadine: a double‐blind, placebo‐controlled trial. Allergy 2000;55(5):484‐8. - PubMed
Roumana 2009 {published data only}
-
- Roumana A, Pitsios C, Vartholomaios S, Kompoti E, Kontou‐Fili K. The safety of initiating Hymenoptera immunotherapy at 1 mug of venom extract. Journal of Allergy and Clinical Immunology 2009;124(2):379‐81. - PubMed
Spertini 2000 {published data only}
-
- Spertini F, Fellrath JM, Kettner A, Dufour N, Frigerio C, Schneeberger D, et al. Allergen‐derived long peptide immunotherapy in bee venom hypersensitive patients. Allergologie 2000;23(3):156.
Thurnheer 1983 {published data only}
-
- Thurnheer U, Müller U, Stoller R, Lanner A, Hoigné R. Venom immunotherapy in hymenoptera sting allergy. Comparison of rush and conventional hyposensitization and observations during long‐term treatment. Allergy 1983;38(7):465‐73. - PubMed
Wohrl 2007 {published data only}
-
- Wöhrl S, Gamper S, Hemmer W, Heinze G, Stingl G, Kinaciyan T. Premedication with montelukast reduces local reactions of allergen immunotherapy. International Archives of Allergy and Immunology 2007;144(2):137‐42. - PubMed
Zubrinich 2010 {published data only}
-
- Zubrinich C, Weber E, Stirling R, Puy R, O'Hehir R, Douglass J. Omalizumab for anaphylaxis during hymenopteravenom immunotherapy [Poster 60]. Australasian Society of Clinical Immunology and Allergy (ASCIA) 21st Annual Scientific Meeting, 1‐3 September 2010, Queensland. Internal Medicine Journal 2010;40(S4):20.
Additional references
Akdis 2007
-
- Akdis M, Akdis CA. Mechanisms of allergen‐specific immunotherapy. Journal of Allergy and Clinical Immunology 2007;119(4):780‐91. - PubMed
Antonicelli 2002
-
- Antonicelli L, Bilo MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Current Opinion in Allergy and Clinical Immunology 2002;2(4):341‐6. - PubMed
Bilo 2009
-
- Bilo MB, Bonifazi F. The natural history and epidemiology of insect venom allergy: clinical implications. Clinical and Experimental Allergy 2009;39(10):1467‐76. - PubMed
Bilo 2005
-
- Bilo BM, Rueff F, Mosbech H, Bonifazi F, Oude Elberink JNG, EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy 2005;60(11):1339‐49. - PubMed
Bonadonna 2009
-
- Bonadonna P, Perbellini O, Passalacqua G, Caruso B, Colarossi S, Dal Fior D, et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. Journal of Allergy and Clinical Immunology 2009;123(3):680‐6. - PubMed
Bonifazi 2005
-
- Bonifazi F, Jutel M, Bilo BM, Birnbaum J, Muller U, EACCI Interest Group on Insect Venom Hypersensitivity. Prevention and treatment of Hymenoptera venom allergy: guidelines for clinical practice. Allergy 2005;60(12):1459‐70. - PubMed
Brown 2004
-
- Brown SG. Clinical features and severity grading of anaphylaxis. Journal of Allergy & Clinical Immunology 2004;114(2):371‐6. - PubMed
Calderon 2011
-
- Calderon MA, Boyle RJ, Penagos M, Sheikh A. Immunotherapy: The Meta‐Analyses. What have we learned?. Immunology and Allergy Clinics of North America 2011;31(2):159‐73. - PubMed
Carballada 2003
-
- Carballada F, Martin S, Boquete M. High efficacy and absence of severe systemic reactions after venom immunotherapy. Journal of Investigative Allergology and Clinical Immunology 2003;13(1):43‐9. - PubMed
Carballada 2009
-
- Carballada FJ, Crehuet AM, Manjon HA, Torre F, Boquete M. Hymenoptera venom allergy: characterisitics, tolerance and efficacy of immunotherapy in the paediatric population. Allergologia et Immunopathologia 2009;37(3):111‐5. - PubMed
Carballada 2010
-
- Carballada F, Boquete M, Nunez R, Lombardero M, Torre F. Follow‐up of venom immunotherapy based on conventional techniques and monitoring of immunoglobulin E to individual venom allergens. Journal of Investigative Allergology and Clinical Immunology 2010;20(6):506‐13. - PubMed
Clark 2007
-
- Clark S, Camargo CA Jr. Epidemiology of anaphylaxis. Immunology and Allergy Clinics of North America 2007;27(2):145‐63. - PubMed
Ewan 2001
-
- Ewan PW. New insight into immunological mechanisms of venom immunotherapy. Current Opinion in Allergy and Clinical Immunology 2001;1(4):367‐74. - PubMed
Freeman 1992
-
- Freeman TM, Hylander R, Ortiz A, Martin ME. Imported fire ant immunotherapy: effectiveness of whole body extracts. Journal of Allergy & Clinical Immunology 1992;90(2):210‐15. - PubMed
Fricker 1997
-
- Fricker M, Helbling A, Schwartz L, Muller U. Hymenoptera sting anaphylaxis and urticaria pigmentosa: clinical findings and results of venom immunotherapy in ten patients. Journal of Allergy and Clinical Immunology 1997;100(1):11‐15. - PubMed
Golden 2004
-
- Golden DB, Kagey‐Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Outcomes of allergy to insect stings in children, with and without venom immunotherapy. New England Journal of Medicine 2004;351(7):668‐74. - PubMed
Golden 2005
-
- Golden DB. Insect sting allergy and venom immunotherapy: a model and a mystery. Journal of Allergy & Clinical Immunology 2005;115(3):439‐47. - PubMed
Golden 2011
-
- Golden DB, Moffit J, Nicklas RA, Freeman T, Graft DF, Reisman RE, et al. Stinging insect hypersensitivity: a practice parameter update 2011. Journal of Allergy and Clinical Immunology 2011;127(4):852‐4. - PubMed
Haeberli 2003
-
- Haeberli G, Bronnimann M, Hunziker T, Muller U. Elevated basal serum tryptase and hymenoptera venom allergy: relation to severity of sting reactions and to safety and efficacy of venom immunotherapy. Clinical and Experimental Allergy 2003;33(9):1216‐20. - PubMed
Higgins 2009
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hockenhull 2012
Kalogeromitros 2010
-
- Kalogeromitros D, Makris M, Koti I, Chliva C, Mellios A, Avgerinou G. A simple 3‐day 'rush' venom immunotherapy protocol: documentation of safety. Allergologia et Immunopathologia 2010;38(2):69‐73. - PubMed
Krishna 2011
-
- Krishna MT, Ewan PW, Diwakar L, Durham SR, Frew AJ, Leech SC, et al. Diagnosis and management of hymenoptera venom allergy: British Society for Allergy and Clinical Immunology (BSACI) guidelines. Clinical & Experimental Allergy 2011;41(9):1201‐20. - PubMed
Liew 2009
-
- Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. Journal of Allergy and Clinical Immunology 2009;123(2):434‐42. - PubMed
Moffit 2004
-
- Moffitt JE, Golden DB, Reisman RE, Lee R, Nicklas R, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update. Journal of Allergy and Clinical Immunology 2004;114(4):869‐86. - PubMed
Mosbech 2000
-
- Mosbech H, Muller U. Side‐effects of insect venom immunotherapy: results from an EAACI multicenter study. Allergy 2000;55(11):1005‐10. - PubMed
Mueller 1966
-
- Mueller, HL. Diagnosis and treatment of insect sensitivity. Journal of Asthma Research 1966;3(4):331‐3. - PubMed
Muller 1989
-
- Muller U, Helbling A, Bischof M. Predictive value of venom‐specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy 1989;44(6):412‐8. - PubMed
Muller 2005
-
- Muller UR. Bee venom allergy in beekeepers and their family members. Current Opinion in Allergy and Clinical Immunology 2005;5(4):343‐7. - PubMed
Oude Elberink 2002
-
- Oude Elberink JNG, Monchy JG, Golden DB, Brouwer JL, Guyatt GH, Dubois AEJ. Development and validation of a health‐related quality‐of‐life questionnaire in patients with yellow jacket allergy. Journal of Allergy and Clinical Immunology 2002;109(1):162‐70. - PubMed
Oude Elberink 2003
-
- Oude Elberink JNG, Dubois AEJ. Quality of life in insect venom allergic patients. Current Opinion in Allergy and Clinical Immunology 2003;3(4):287‐93. - PubMed
Pumphrey 2000
-
- Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clinical and Experimental Allergy 2000;30(8):1144‐50. - PubMed
Radulovic 2011
-
- Radulovic S, Wilson D, Calderon M, Durham S. Systematic reviews of sublingual immunotherapy. Allergy 2011;66(6):740‐52. - PubMed
Ramirez 1981
-
- Ramirez DA, Londono S, Evans IRD. Adverse reactions to venom immunotherapy. Annals of Allergy 1981;47(6):435‐9. - PubMed
Rueff 2009
-
- Rueff F, Przybilla B, Bilo MB, Muller U, Scheipl F, Aberer W, et al. Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: importance of baseline serum tryptase‐a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom Hypersensitivity. Journal of Allergy and Clinical Immunology 2009;124(5):1047‐54. - PubMed
Sanchez‐Machin 2010
-
- Sanchez‐Machin I, Moreno C, Gonzalez R, Iglesias‐Souto J, Perez E, Matheu, V. Safety of a 2‐visit cluster schedule of venom immunotherapy in outpatients at risk of life‐threatening anaphylaxis. Journal of Investigative Allergology and Clinical Immunology 2010;20(1):91‐2. - PubMed
Schiavino 2004
-
- Schiavino D, Nucera E, Pollastrini E, Pasquale T, Buonomo A, Bartolozzi F, et al. Specific ultrarush desensitisation in hymenoptera venom‐allergic patients. Annals of Allergy Asthma and Immunology 2004;92(4):409‐13. - PubMed
Senti 2006
-
- Senti G, Johansen P, Oliver R, Prinz Vavricka BM, Graf N, Wuthrich B, et al. A cutaneous allergen neutralisation test that correlates with the duration of venom immunotherapy. Internal Archives of Allergy and Immunology 2006;141(4):377‐83. - PubMed
Watanabe 2010
Wilson 1994
-
- Wilson AB, Deighton J, Lachmann PJ, Ewan PW. A comparative study of IgG subclass antibodies in patients allergic to wasp or bee venom. Allergy 1994;49(4):272‐80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

