Safety of regular formoterol or salmeterol in children with asthma: an overview of Cochrane reviews
- PMID: 23076961
- PMCID: PMC4022036
- DOI: 10.1002/14651858.CD010005.pub2
Safety of regular formoterol or salmeterol in children with asthma: an overview of Cochrane reviews
Abstract
Background: Two large surveillance studies in adults with asthma have found an increased risk of asthma-related mortality in those who took regular salmeterol as monotherapy in comparison to placebo or regular salbutamol. No similar sized surveillance studies have been carried out in children with asthma, and we remain uncertain about the comparative safety of regular combination therapy with either formoterol or salmeterol in children with asthma.
Objectives: We have used the paediatric trial results from Cochrane systematic reviews to assess the safety of regular formoterol or salmeterol, either as monotherapy or as combination therapy, in children with asthma.
Methods: We included Cochrane reviews relating to the safety of regular formoterol and salmeterol from a search of the Cochrane Database of Systematic Reviews conducted in May 2012, and ran updated searches for each of the reviews. These were independently assessed. All the reviews were assessed for quality using the AMSTAR tool. We extracted the data relating to children from each review and from new trials found in the updated searches (including risks of bias, study characteristics, serious adverse event outcomes, and control arm event rates).The safety of regular formoterol and salmeterol were assessed directly from the paediatric trials in the Cochrane reviews of monotherapy and combination therapy with each product. Then monotherapy was indirectly compared to combination therapy by looking at the differences between the pooled trial results for monotherapy and the pooled results for combination therapy. The comparative safety of formoterol and salmeterol was assessed using direct evidence from trials that randomised children to each treatment; this was combined with the result of an indirect comparison of the combination therapy trials, which represents the difference between the pooled results of each product when randomised against inhaled corticosteroids alone.
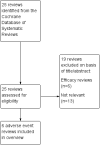
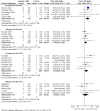
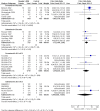
Main results: We identified six high quality, up to date Cochrane reviews. Four of these related to the safety of regular formoterol or salmeterol (as monotherapy or combination therapy) and these included 19 studies in children. We added data from two recent studies on salmeterol combination therapy in 689 children which were published after the relevant Cochrane review had been completed, making a total of 21 trials on 7474 children (from four to 17 years of age). The two remaining reviews compared the safety of formoterol with salmeterol from trials randomising participants to one or other treatment, but the reviews only included a single trial in children in which there were 156 participants.Only one child died across all the trials, so impact on mortality could not be assessed.We found a statistically significant increase in the odds of suffering a non-fatal serious adverse event of any cause in children on formoterol monotherapy (Peto odds ratio (OR) 2.48; 95% confidence interval (CI) 1.27 to 4.83, I(2) = 0%, 5 trials, N = 1335, high quality) and smaller increases in odds which were not statistically significant for salmeterol monotherapy (Peto OR 1.30; 95% CI 0.82 to 2.05, I(2) = 17%, 5 trials, N = 1333, moderate quality), formoterol combination therapy (Peto OR 1.60; 95% CI 0.80 to 3.28, I(2) = 32%, 7 trials, N = 2788, moderate quality) and salmeterol combination therapy (Peto OR 1.20; 95% CI 0.37 to 2.91, I(2) = 0%, 5 trials, N = 1862, moderate quality).We compared the pooled results of the monotherapy and combination therapy trials. There was no significant difference between the pooled ORs of children with a serious adverse event (SAE) from long-acting beta(2)-agonist beta agonist (LABA) monotherapy (Peto OR 1.60; 95% CI 1.10 to 2.33, 10 trials, N = 2668) and combination trials (Peto OR 1.50; 95% CI 0.82 to 2.75, 12 trials, N = 4,650). However, there were fewer children with an SAE in the regular inhaled corticosteroid (ICS) control group (0.7%) than in the placebo control group (3.6%). As a result, there was an absolute increase of an additional 21 children (95% CI 4 to 45) suffering such an SAE of any cause for every 1000 children treated over six months with either regular formoterol or salmeterol monotherapy, whilst for combination therapy the increased risk was an additional three children (95% CI 1 fewer to 12 more) per 1000 over three months.We only found a single trial in 156 children comparing the safety of regular salmeterol to regular formoterol monotherapy, and even with the additional evidence from indirect comparisons between the combination formoterol and salmeterol trials, the CI around the effect on SAEs is too wide to tell whether there is a difference in the comparative safety of formoterol and salmeterol (OR 1.26; 95% CI 0.37 to 4.32).
Authors' conclusions: We do not know if regular combination therapy with formoterol or salmeterol in children alters the risk of dying from asthma.Regular combination therapy is likely to be less risky than monotherapy in children with asthma, but we cannot say that combination therapy is risk free. There are probably an additional three children per 1000 who suffer a non-fatal serious adverse event on combination therapy in comparison to ICS over three months. This is currently our best estimate of the risk of using LABA combination therapy in children and has to be balanced against the symptomatic benefit obtained for each child. We await the results of large on-going surveillance studies to further clarify the risks of combination therapy in children and adolescents with asthma.The relative safety of formoterol in comparison to salmeterol remains unclear, even when all currently available direct and indirect trial evidence is combined.
Conflict of interest statement
Chris Cates authored the included systematic reviews on the adverse events of long‐acting beta2‐agonists in adults and children, and was not involved in the assessment of the quality of the reviews.
Figures






Update of
- doi: 10.1002/14651858.CD010005
References
References to included reviews
Cates 2008
Cates 2009a
Cates 2009b
Cates 2010
-
- Cates Christopher J, Lasserson Toby J. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2010, issue 1. [DOI: 10.1002/14651858.CD007694.pub2; CD007694] - DOI - PMC - PubMed
Cates 2012a
Additional references
Altman 2003
Anderson 2008
-
- Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008;372(9643):1107‐19. - PubMed
Boushey 1980
-
- Boushey HA, Holtzman MJ, Sheller JR, Nadel JA. Bronchial hyperreactivity. American Review of Respiratory Disease 1980;121(2):389‐413. - PubMed
Bradburn 2007
-
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007;26(1):53‐77. - PubMed
Bucher 1997
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. - PubMed
Castle 1993
Cates 2011
-
- Cates CJ. Safety of tiotropium. BMJ 2011;342:d2970. - PubMed
Chan 2004
-
- Chan A‐W, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. - PubMed
Chan 2004a
Chowdhury 2011
-
- Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding LABAs to inhaled corticosteroids for treating asthma. The New England Journal of Medicine 2011;364(26):2473‐5. - PubMed
Cockcroft 2006
-
- Cockcroft D. Airway hyper‐responsiveness as a determinant of the early asthmatic response to inhaled allergen. Journal of Asthma 2006;43(3):175‐8. - PubMed
Ducharme 2010
-
- Ducharme Francine M, Ni Chroinin M, Greenstone I, Lasserson Toby J. Addition of long‐acting beta2‐agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2010, issue 5. [DOI: 10.1002/14651858.CD005535.pub2] - DOI - PMC - PubMed
Ducharme 2011
-
- Ducharme Francine M, Lasserson Toby J, Cates Christopher J. Addition to inhaled corticosteroids of long‐acting beta2‐agonists versus anti‐leukotrienes for chronic asthma. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2011, issue 5. [DOI: 10.1002/14651858.CD003137.pub4] - DOI - PubMed
GINA 2011
-
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. Available from: http://www.ginasthma.org/. 2011.
ICH E2A 1995
-
- Expert Working Group (Efficacy) of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Clinical safety data management: definitions and standards for expedited reporting. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guid... (accessed July 2012). 1995.
Ioannidis 2001
-
- Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA 2001;285(4):437‐43. - PubMed
Ioannidis 2004
-
- Ioannidis JPA, Evans SJW, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. CONSORT Group. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. - PubMed
Li 2010
-
- Li JS, Qaqundah PY, Weinstein SF, LaForce CF, Ellsworth AV, Ortega HG, et al. Fluticasone propionate/salmeterol combination in children with asthma: Key cardiac and overall safety results. Clinical Research and Regulatory Affairs 2010;27(3):87‐95.
Lougheed 2010
McMahon 2011
-
- McMahon AW, Levenson MS, McEvoy BW, Mosholder AD, Murphy D. Age and risks of FDA–approved long‐acting β2‐adrenergic receptor agonists. Pediatrics 2011;128(5):e1147‐54. - PubMed
NCT01192178
-
- GlaxoSmithKline. A Randomized, Double‐Blind, Parallel Group Study of FSC 100/50 and FP 100, both twice daily, in a Pediatric Population during the Fall Viral Season [ADA113872]. http://www.gsk‐clinicalstudyregister.com/.
NCT01444430
-
- AstraZeneca. A 6 Month Safety Study Comparing Symbicort With Inhaled Corticosteroid Only in Asthmatic Adults and Adolescents. ClinicalTrials.gov 2012 (accessed June 2012).
NCT01462344
-
- GlaxoSmithKline. 6‐month Safety and Benefit Study of ADVAIR in Children 4‐11 Years Old (VESTRI). ClinicalTrials.gov 2012 (accessed 29/06/2012).
NCT01471340
-
- Merck. A Serious Asthma Outcome Study With Mometasone Furoate/Formoterol Versus Mometasone Furoate in Asthmatics 12 Years and Over (P06241 AM3) (SPIRO). ClinicalTrials.gov 2012 (accessed June 2012).
NCT01475721
-
- GlaxoSmithKline. SAS115359: A 6‐month Study to Assess the Safety and Benefit of Inhaled Fluticasone Propionate/Salmeterol Combination Compared With Inhaled Fluticasone Propionate in the Treatment of Adolescents and Adults (12 Years of Age and Older) With Asthma. (AUSTRI). ClinicalTrials.gov 2012 (accessed June 2012).
Nelson 2006
-
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM, SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006;129(1):15‐26. - PubMed
Ni Chroinin 2009
Phillips 1990
-
- Phillips GD, Finnerty JP, Holgate ST. Comparative protective effect of the inhaled beta‐2‐agonist salbutamol (albuterol) on bronchoconstriction provoked by histamine, methacholine, and adenosine 5'‐monophosphate in asthma. Journal of Allergy and Clinical Immunology 1990;85(4):755‐62. - PubMed
RevMan 5 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Salpeter 2006
-
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta‐analysis: effect of long‐acting beta‐agonists on severe asthma exacerbations and asthma‐related deaths. Annals of Internal Medicine 2006;144(12):904‐12. - PubMed
Senn 1997
-
- Senn SJ, Lillienthal J, Patalano F, Till D. An incomplete blocks cross‐over in asthma: a case study in collaboration. In: Vollmar J, Hothorn LA editor(s). Cross‐over Clinical Trials. Stuttgart: Fischer, 1997:3‐26.
Shea 2007
SIGN/BTS 2012
-
- Scottish Intercollegiate Guidelines Network/British Thoracic Society. British guideline on the management of asthma: a national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network, 2008 (revised 2012).
Tattersfield 2006
-
- Tattersfield AE. Current issues with beta2‐adrenoceptor agonists: historical background. Clinical Reviews in Allergy and Immunology 2006;31(2‐3):107‐18. - PubMed
Van Noord 1996
-
- Noord JA, Smeets JJ, Raaijmakers JAM, Bommer AM, Maesen FPV. Salmeterol versus formoterol in patients with moderately severe asthma: onset and duration of action. European Respiratory Journal 1996;9(8):1684‐8. - PubMed
Visual Rx 2012
-
- Visual Rx. www.nntonline.net/visualrx/ Accessed June 2012.
Weinberger 2006
-
- Weinberger M, Abu‐Hasan M. Life threatening asthma during treatment with salmeterol. The New England Journal of Medicine 2006;355:852‐3. - PubMed
Whittington 2004
-
- Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363(9418):1341‐5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

