Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles
- PMID: 23077170
- PMCID: PMC4435432
- DOI: 10.1095/biolreprod.112.102467
Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles
Abstract
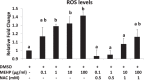
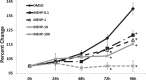
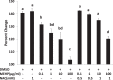
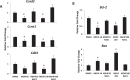
Mono-(2-ethylhexyl) phthalate (MEHP) is the active metabolite of the most commonly used plasticizer, di-(2-ethylhexyl) phthalate, and is considered to be a reproductive toxicant. However, little is known about the effects of MEHP on ovarian antral follicles. Thus, the present study tested the hypothesis that MEHP inhibits follicle growth via oxidative stress pathways. The data indicate that MEHP increases reactive oxygen species (ROS) levels and inhibits follicle growth in antral follicles, whereas N-acetylcysteine (NAC; an antioxidant) restores ROS levels to control levels and rescues follicles from MEHP-induced inhibition of follicle growth. To further analyze the mechanism by which MEHP induces oxidative stress and inhibits follicle growth, the expression and activities of various key antioxidant enzymes (copper/zinc superoxide dismutase [SOD1], glutathione peroxidase [GPX], and catalase [CAT]) and the expression of key cell-cycle regulators (Ccnd2, Ccne1, and Cdk4) and apoptotic regulators (Bcl-2 and Bax) were compared in control and MEHP-treated follicles. The data indicate that MEHP inhibits the expression and activities of SOD1 and GPX; does not inhibit Cat expression; inhibits the expression of Ccnd2, Ccne1, Cdk4, and Bcl-2; but increases the expression of Bax compared to controls. Furthermore, NAC blocks these toxic effects of MEHP. Collectively, these data suggest that MEHP induces oxidative stress by disrupting the activities of antioxidant enzymes. This may lead to decreased expression of cell-cycle regulators and antiapoptotic regulators and increased expression of proapoptotic factors, which then may lead to inhibition of follicle growth.
Figures



Similar articles
-
Di (2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway.Toxicol Appl Pharmacol. 2012 Jan 15;258(2):288-95. doi: 10.1016/j.taap.2011.11.008. Epub 2011 Nov 23. Toxicol Appl Pharmacol. 2012. PMID: 22155089 Free PMC article.
-
Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro.Toxicol Appl Pharmacol. 2010 Jan 15;242(2):224-30. doi: 10.1016/j.taap.2009.10.011. Epub 2009 Oct 27. Toxicol Appl Pharmacol. 2010. PMID: 19874833 Free PMC article.
-
Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles.Toxicol Appl Pharmacol. 2015 Apr 1;284(1):42-53. doi: 10.1016/j.taap.2015.02.010. Epub 2015 Feb 18. Toxicol Appl Pharmacol. 2015. PMID: 25701202 Free PMC article.
-
Antioxidants in mitigating phthalate-induced male reproductive toxicity: A comprehensive review.Chemosphere. 2024 Sep;364:143297. doi: 10.1016/j.chemosphere.2024.143297. Epub 2024 Sep 6. Chemosphere. 2024. PMID: 39245218 Review.
-
N-acetyl-cysteine and the control of oxidative stress during in vitro ovarian follicle growth, oocyte maturation, embryo development and cryopreservation.Anim Reprod Sci. 2021 Aug;231:106801. doi: 10.1016/j.anireprosci.2021.106801. Epub 2021 Jul 6. Anim Reprod Sci. 2021. PMID: 34252825 Review.
Cited by
-
The Impact of Di-2-Ethylhexyl Phthalate on Sperm Fertility.Front Cell Dev Biol. 2020 Jun 30;8:426. doi: 10.3389/fcell.2020.00426. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32695775 Free PMC article.
-
The Deep Correlation between Energy Metabolism and Reproduction: A View on the Effects of Nutrition for Women Fertility.Nutrients. 2016 Feb 11;8(2):87. doi: 10.3390/nu8020087. Nutrients. 2016. PMID: 26875986 Free PMC article. Review.
-
Di (2-ethylhexyl) Phthalate Exposure Impairs Growth of Antral Follicle in Mice.PLoS One. 2016 Feb 4;11(2):e0148350. doi: 10.1371/journal.pone.0148350. eCollection 2016. PLoS One. 2016. PMID: 26845775 Free PMC article.
-
Phthalates are detected in the follicular fluid of adolescents and oocyte donors with associated changes in the cumulus cell transcriptome.F S Sci. 2025 Feb;6(1):30-41. doi: 10.1016/j.xfss.2024.10.009. Epub 2024 Nov 7. F S Sci. 2025. PMID: 39515754
-
Pyruvate Regulates the Expression of DLAT to Promote Follicular Growth.Cells. 2025 Mar 17;14(6):444. doi: 10.3390/cells14060444. Cells. 2025. PMID: 40136693 Free PMC article.
References
-
- Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health 2007; 210: 623 634. - PubMed
-
- Halden RU. Plastics and health risks. Annu Rev Public Health 2010; 31: 179 194. - PubMed
-
- Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, Saunders M, Skaare JU. Reproductive and developmental toxicity of phthalates. J Toxicol Environ Health B Crit Rev 2009; 12: 225 249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

