Anti-Gr-1 antibody depletion fails to eliminate hepatic myeloid-derived suppressor cells in tumor-bearing mice
- PMID: 23077247
- PMCID: PMC3501895
- DOI: 10.1189/jlb.0212059
Anti-Gr-1 antibody depletion fails to eliminate hepatic myeloid-derived suppressor cells in tumor-bearing mice
Abstract
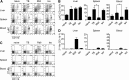
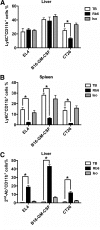
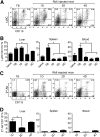
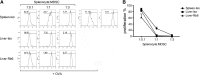
Recent studies show that the liver is a preferred organ for the accumulation of MDSC. In this study, we examined the effect of systemic RB6-8C5 treatment on hepatic MDSC in tumor-bearing mice. EL4 tumor-bearing mice were injected i.p. with RB6-8C5, and hepatic, splenic, and blood MDSCs were analyzed by flow cytometry. Unexpectedly, hepatic MDSC remained in the liver, although RB6-8C5 completely eliminated them from the spleen and peripheral blood 24 h after treatment. Secondary antibody staining confirmed the presence of RB6-8C5-bound MDSC in the liver of mice with s.c. tumors. Similar observations were made in two other (colon and melanoma) tumor models. Whereas RB6-8C5 injection induced cell death of hepatic MDSC, as shown by Annexin V/7-AAD staining, these cells were replaced immediately, leading to a constant, increased frequency of hepatic MDSC. Adoptively transferred MDSC migrated preferentially to the liver after RB6-8C5 treatment, suggesting that hepatic MDSCs are reconstituted rapidly after depletion. Finally, hepatic MDSC remained immunosuppressive despite RB6-8C5 injection. Our study demonstrates that RB6-8C5 is not suitable for depletion of hepatic MDSCs and analysis of their function.
Figures


Similar articles
-
Tumor induced hepatic myeloid derived suppressor cells can cause moderate liver damage.PLoS One. 2014 Nov 17;9(11):e112717. doi: 10.1371/journal.pone.0112717. eCollection 2014. PLoS One. 2014. PMID: 25401795 Free PMC article.
-
Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection.J Immunol. 1994 Feb 15;152(4):1836-46. J Immunol. 1994. PMID: 8120393
-
Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice.J Leukoc Biol. 2008 Jan;83(1):64-70. doi: 10.1189/jlb.0407247. Epub 2007 Sep 20. J Leukoc Biol. 2008. PMID: 17884993
-
Systemic Agonistic Anti-CD40 Treatment of Tumor-Bearing Mice Modulates Hepatic Myeloid-Suppressive Cells and Causes Immune-Mediated Liver Damage.Cancer Immunol Res. 2015 May;3(5):557-66. doi: 10.1158/2326-6066.CIR-14-0182. Epub 2015 Jan 30. Cancer Immunol Res. 2015. PMID: 25637366 Free PMC article.
-
MDSC in autoimmunity.Int Immunopharmacol. 2011 Jul;11(7):789-93. doi: 10.1016/j.intimp.2011.01.026. Epub 2011 Feb 21. Int Immunopharmacol. 2011. PMID: 21310255 Free PMC article. Review.
Cited by
-
Donor myeloid derived suppressor cells (MDSCs) prolong allogeneic cardiac graft survival through programming of recipient myeloid cells in vivo.Sci Rep. 2020 Aug 28;10(1):14249. doi: 10.1038/s41598-020-71289-z. Sci Rep. 2020. PMID: 32859934 Free PMC article.
-
Myeloid-derived suppressor cells in hematological malignancies: friends or foes.J Hematol Oncol. 2019 Oct 22;12(1):105. doi: 10.1186/s13045-019-0797-3. J Hematol Oncol. 2019. PMID: 31640764 Free PMC article. Review.
-
Granulocyte Colony-Stimulating Factor Attenuates Renal Ischemia-Reperfusion Injury by Inducing Myeloid-Derived Suppressor Cells.J Am Soc Nephrol. 2020 Apr;31(4):731-746. doi: 10.1681/ASN.2019060601. Epub 2020 Mar 4. J Am Soc Nephrol. 2020. PMID: 32132198 Free PMC article.
-
Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma.J Hepatol. 2013 Nov;59(5):1007-13. doi: 10.1016/j.jhep.2013.06.010. Epub 2013 Jun 22. J Hepatol. 2013. PMID: 23796475 Free PMC article.
-
Exploring the potential use of Chinese herbs in regulating the inflammatory microenvironment of tumours based on the concept of 'state-target identification and treatment': a scooping review.Chin Med. 2023 Sep 23;18(1):124. doi: 10.1186/s13020-023-00834-5. Chin Med. 2023. PMID: 37742025 Free PMC article. Review.
References
-
- Haile L. A., von Wasielewski R., Gamrekelashvili J., Kruger C., Bachmann O., Westendorf A. M., Buer J., Liblau R., Manns M. P., Korangy F., Greten T. F. (2008) Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology 135, 871–881, 881.e1–881.e5 - PubMed
-
- Haile L. A., Gamrekelashvili J., Manns M. P., Korangy F., Greten T. F. (2010) CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J. Immunol. 185, 203–10 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

