Marked difference in saxitoxin and tetrodotoxin affinity for the human nociceptive voltage-gated sodium channel (Nav1.7) [corrected]
- PMID: 23077250
- PMCID: PMC3497785
- DOI: 10.1073/pnas.1206952109
Marked difference in saxitoxin and tetrodotoxin affinity for the human nociceptive voltage-gated sodium channel (Nav1.7) [corrected]
Erratum in
- Proc Natl Acad Sci U S A. 2012 Dec 26;109(52):21551
Abstract
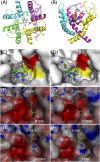
Human nociceptive voltage-gated sodium channel (Na(v)1.7), a target of significant interest for the development of antinociceptive agents, is blocked by low nanomolar concentrations of (-)-tetrodotoxin(TTX) but not (+)-saxitoxin (STX) and (+)-gonyautoxin-III (GTX-III). These findings question the long-accepted view that the 1.7 isoform is both tetrodotoxin- and saxitoxin-sensitive and identify the outer pore region of the channel as a possible target for the design of Na(v)1.7-selective inhibitors. Single- and double-point amino acid mutagenesis studies along with whole-cell electrophysiology recordings establish two domain III residues (T1398 and I1399), which occur as methionine and aspartate in other Na(v) isoforms, as critical determinants of STX and gonyautoxin-III binding affinity. An advanced homology model of the Na(v) pore region is used to provide a structural rationalization for these surprising results.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology International Union of Pharmacology. XXXIX. Compendium of voltage-gated ion channels: Sodium channels. Pharmacol Rev. 2003;55(4):575–578. - PubMed
-
- Novella SP, Hisama FM, Dib-Hajj SD, Waxman SG. A case of inherited erythromelalgia. Nat Clin Pract Neurol. 2007;3(4):229–234. - PubMed
-
- Williams BS, et al. Characterization of a new class of potent inhibitors of the voltage-gated sodium channel Nav1.7. Biochemistry. 2007;46(50):14693–14703. - PubMed
-
- Schmalhofer WA, et al. ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol Pharmacol. 2008;74(5):1476–1484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

