Role of interferon regulatory factor 3-mediated apoptosis in the establishment and maintenance of persistent infection by Sendai virus
- PMID: 23077293
- PMCID: PMC3536409
- DOI: 10.1128/JVI.01853-12
Role of interferon regulatory factor 3-mediated apoptosis in the establishment and maintenance of persistent infection by Sendai virus
Abstract
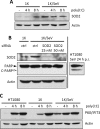
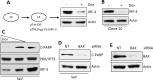
Infection of cultured cells by paramyxoviruses causes cell death, mediated by a newly discovered apoptotic pathway activated by virus infection. The key proapoptotic protein in this pathway is interferon regulatory factor 3 (IRF-3), which upon activation by virus infection binds BAX, translocates it to mitochondria, and triggers apoptosis. When IRF-3-knockdown cells were infected with Sendai virus (SeV), persistent infection (PI) was established. The PI cells produced infectious SeV continuously and constitutively expressed many innate immune genes. Interferon signaling was blocked in these cells. The elevated levels of IRF-3-driven genes in the PI cells indicated that the amount of residual IRF-3 activated by endogenous SeV was high enough to drive the transcriptional effects of IRF-3 but too low to trigger its apoptotic activity. We confirmed this IRF-3 threshold idea by generating a tetracycline (Tet)-inducible cell line for IRF-3 expression, which enabled us to express various levels of IRF-3. PI could be established in the Tet-off cell line, and as expected, when doxycycline was withdrawn, the cells underwent apoptosis. Finally, we tested for PI establishment in 12 mouse embryo fibroblasts by natural selection. Eleven lines became persistently infected; although seven out of them had low IRF-3 levels, four did not. When one of the latter four was further analyzed, we observed that it expressed a very low level of caspase 3, the final executor protease of the apoptotic pathway. These results demonstrated that SeV PI can arise from infection of normal wild-type cells, but only if they can find a way to impair the IRF-3-dependent apoptotic pathway.
Figures





References
-
- Griffin DE, Hardwick JM. 1997. Regulators of apoptosis on the road to persistent alphavirus infection. Annu. Rev. Microbiol. 51:565–592 - PubMed
-
- Sarkar SN, Sen GC. 2004. Novel functions of proteins encoded by viral stress-inducible genes. Pharmacol. Ther. 103:245–259 - PubMed
-
- Colonna M. 2007. TLR pathways and IFN-regulatory factors: to each its own. Eur. J. Immunol. 37:306–309 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

