Complex intramolecular mechanics of G-actin--an elastic network study
- PMID: 23077498
- PMCID: PMC3471905
- DOI: 10.1371/journal.pone.0045859
Complex intramolecular mechanics of G-actin--an elastic network study
Abstract
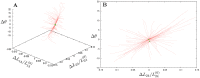
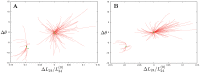
Systematic numerical investigations of conformational motions in single actin molecules were performed by employing a simple elastic-network (EN) model of this protein. Similar to previous investigations for myosin, we found that G-actin essentially behaves as a strain sensor, responding by well-defined domain motions to mechanical perturbations. Several sensitive residues within the nucleotide-binding pocket (NBP) could be identified, such that the perturbation of any of them can induce characteristic flattening of actin molecules and closing of the cleft between their two mobile domains. Extending the EN model by introduction of a set of breakable links which become effective only when two domains approach one another, it was observed that G-actin can possess a metastable state corresponding to a closed conformation and that a transition to this state can be induced by appropriate perturbations in the NBP region. The ligands were roughly modeled as a single particle (ADP) or a dimer (ATP), which were placed inside the NBP and connected by elastic links to the neighbors. Our approximate analysis suggests that, when ATP is present, it stabilizes the closed conformation of actin. This may play an important role in the explanation why, in the presence of ATP, the polymerization process is highly accelerated.
Conflict of interest statement
Figures
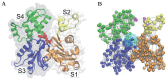


 (grey bead), connected by an elastic link. The ADP is elastically connected to its neighbors and the phosphate is elastically linked to the three sensitive nodes (red beads).
(grey bead), connected by an elastic link. The ADP is elastically connected to its neighbors and the phosphate is elastically linked to the three sensitive nodes (red beads).
 is visualized as a small red bead; ATP is modeled as a dimer consisting of ADP and Pi. Magenta-colored beads indicate residues between which additional breakable links can become established. Such breakable links are shown by solid magenta lines, if they are actually present, and by dashed lines if they are broken.
is visualized as a small red bead; ATP is modeled as a dimer consisting of ADP and Pi. Magenta-colored beads indicate residues between which additional breakable links can become established. Such breakable links are shown by solid magenta lines, if they are actually present, and by dashed lines if they are broken.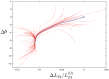
References
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, et al.. (2008) Molecular Biology of the Cell. Garland Press, 5th edition.
-
- Korn ED, Carlier MF, Pantaloni D (1987) Actin polymerization and ATP hydrolysis. Science 238: 638–644. - PubMed
-
- Otterbein LR, Graceffa P, Dominguez R (2001) The crystal structure of uncomplexed actin in the ADP state. Science 293: 708–11. - PubMed
-
- Graceffa P, Dominguez R (2003) Crystal structure of monomeric actin in the ATP state. Structural basis of nucleotide-dependent actin dynamics. The Journal of Biological Chemistry 278: 34172–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

