Patient-Reported Outcomes vs. Clinician Symptom Reporting During Chemoradiation for Rectal Cancer
- PMID: 23077685
- PMCID: PMC3433260
Patient-Reported Outcomes vs. Clinician Symptom Reporting During Chemoradiation for Rectal Cancer
Abstract
Background: Pelvic radiotherapy with concurrent 5-fluorouracil-based chemotherapy is a component of standard therapy for patients with T3/T4 or node-positive rectal cancer and may be associated with acute gastrointestinal toxicity. In this retrospective study, we sought to compare patient-reported outcomes (PROs) with clinician reports of acute symptoms experienced by rectal cancer patients receiving chemoradiation.
Patients and methods: Charts of 199 patients with rectal cancer who received chemoradiation at some point from November 2006 through February 2011 were reviewed. Clinicians assessed toxicity weekly using Common Terminology for Clinical Adverse Events version 3.0, and, beginning in September 2009, the patients reported symptoms weekly, using the 7-item Bowel Problems Scale. One hundred ninety-seven patients with at least 1 clinician or patient assessment were eligible for the study. We used descriptive statistics to compare patient and clinician assessments in a subgroup of 65 patients (paired group) who had at least 1 patient and clinician assessment on the same day. Cohen's κ coefficient was used to evaluate agreement between the patients and the clinicians.
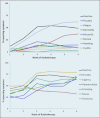
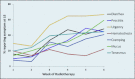
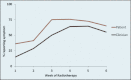
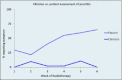
Results: The patients reported diarrhea and proctitis more often than clinicians reported them throughout treatment. Uncorrected agreement for diarrhea and proctitis was 82% and 72%, respectively. Cohen's κ was .64 for diarrhea, indicating moderate agreement, and .22 for proctitis, indicating only slight agreement.
Conclusions: Our findings suggest a discrepancy between clinician and PRO reports. Further study may discern potential benefits of collecting PROs in prospective studies and in clinical practice.
Figures
References
-
- Trotti A, Colevas AD, Setser A, et al. : Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol 25:5121–5127, 2007 - PubMed
-
- Gondek K, Sagnier PP, Gilchrist K, et al. : Current status of patient-reported outcomes in industry-sponsored oncology clinical trials and product labels. J Clin Oncol 25:5087–5093, 2007 - PubMed
-
- Garcia SF, Cella D, Clauser SB, et al. : Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol 25:5106–5112, 2007 - PubMed
-
- Langendijk H, Aaronson NK, de Jong JM, et al. : The prognostic impact of quality of life assessed with the EORTC QLQ-C30 in inoperable non-small cell lung carcinoma treated with radiotherapy. Radiother Oncol 55:19–25, 2000 - PubMed
-
- Fang FM, Liu YT, Tang Y, et al. : Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer 100:425–432, 2004 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
