Safety and efficacy of fluticasone/formoterol combination therapy in adolescent and adult patients with mild-to-moderate asthma: a randomised controlled trial
- PMID: 23078148
- PMCID: PMC3502550
- DOI: 10.1186/1471-2466-12-67
Safety and efficacy of fluticasone/formoterol combination therapy in adolescent and adult patients with mild-to-moderate asthma: a randomised controlled trial
Abstract
Background: This study investigated the efficacy and safety of a new asthma therapy combining fluticasone propionate and formoterol fumarate (fluticasone/formoterol; flutiform®), administered twice daily (b.i.d.) via a single aerosol inhaler, compared with its individual components administered separately and placebo, in patients with mild-to-moderate asthma.
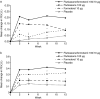
Methods: Patients aged ≥ 12 years were evenly randomised to 12 weeks of treatment with fluticasone/formoterol (100/10 μg b.i.d.), fluticasone (100 μg b.i.d.), formoterol (10 μg b.i.d.), or placebo, in this double-blind, parallel group, multicentre study. The three co-primary endpoints were: a) change in forced expiratory volume in the first second (FEV(1)) from morning pre-dose at baseline to pre-dose at week 12 for the comparison with formoterol; b) change in FEV(1) from morning pre-dose at baseline to 2 hours post-dose at week 12 for the comparison with fluticasone, and c) time to discontinuation due to lack of efficacy from baseline to week 12 for the comparison with placebo. Safety was assessed based on adverse events, clinical laboratory tests and vital sign evaluations.
Results: Statistically significant differences were demonstrated for all the three co-primary endpoints. Fluticasone/formoterol combination therapy showed significantly greater improvements from baseline to end of study in the change in pre-dose FEV(1) compared with formoterol (Least Squares (LS) mean treatment difference: 0.101 L; 95% Confidence Interval (CI): 0.002, 0.199; p = 0.045) and the change in pre-dose compared with 2 hours post-dose FEV(1) versus fluticasone (LS mean treatment difference: 0.200 L; 95% CI: 0.109, 0.292; p < 0.001). The time to discontinuation due to lack of efficacy was significantly longer for patients in the combination therapy group compared with those receiving placebo (p = 0.015). Overall, the results from multiple secondary endpoints assessing lung function, asthma symptoms, and rescue medication use supported the superior efficacy of the combination product compared with fluticasone, formoterol, and placebo. The fluticasone/formoterol combination therapy had a good safety and tolerability profile over the 12 week treatment period.
Conclusions: Fluticasone/formoterol had a good safety and tolerability profile and showed statistically superior efficacy for the three co-primary endpoints compared to fluticasone, formoterol, and placebo, in adolescents and adults with mild-to-moderate asthma. EudraCT number: 2007-002866-36; US NCT number: NCT00393991.
Figures

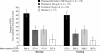
References
-
- Pauwels RA, Lofdahl CL, Postma DS, Tattersfield AE, O’Byrne P, Barnes PJ, Ullman A. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Eng J Med. 1997;337:1405–1411. - PubMed
-
- GINA report, global strategy for asthma management and prevention. [http://www.ginasthma.com/] 2009 Accessed January 2011. - PubMed
-
- Naedele-Risha R, Dorinsky P, Craig TJ. Dual components of optimal asthma therapy: scientific and clinical rationale for the use of long-acting b-agonists with inhaled corticosteroids. J Am Osteopath Assoc. 2001;1001:526–533. - PubMed

