Discovery and validation of SIRT2 inhibitors based on tenovin-6: use of a ¹H-NMR method to assess deacetylase activity
- PMID: 23079492
- PMCID: PMC6268290
- DOI: 10.3390/molecules171012206
Discovery and validation of SIRT2 inhibitors based on tenovin-6: use of a ¹H-NMR method to assess deacetylase activity
Abstract
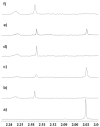
The search for potent and selective sirtuin inhibitors continues as chemical tools of this type are of use in helping to assign the function of this interesting class of deacetylases. Here we describe SAR studies starting from the unselective sirtuin inhibitor tenovin-6. These studies identify a sub-micromolar inhibitor that has increased selectivity for SIRT2 over SIRT1 compared to tenovin-6. In addition, a ¹H-NMR-based method is developed and used to validate further this class of sirtuin inhibitors. A thermal shift analysis of SIRT2 in the presence of tenovin-6, -43, a control tenovin and the known SIRT2 inhibitor AGK2 is also presented.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

