The C-terminal helix of Bcl-x(L) mediates Bax retrotranslocation from the mitochondria
- PMID: 23079612
- PMCID: PMC3554327
- DOI: 10.1038/cdd.2012.131
The C-terminal helix of Bcl-x(L) mediates Bax retrotranslocation from the mitochondria
Abstract
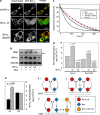
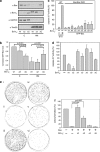
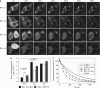
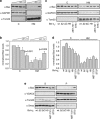
The proapoptotic Bcl-2 protein Bax can commit a cell to apoptosis by translocation from the cytosol to the mitochondria and permeabilization of the outer mitochondrial membrane. Prosurvival Bcl-2 family members, such as Bcl-x(L), control Bax activity. Bcl-x(L) recognizes Bax after a conformational change in the N-terminal segment of Bax on the mitochondria and retrotranslocates it back into the cytoplasm, stabilizing the inactive form of Bax. Here we show that Bax retrotranslocation depends on the C-terminal helix of Bcl-x(L). Deletion or substitution of this segment reduces Bax retrotranslocation and correlates with the accumulation of GFP-tagged or endogenous Bax on the mitochondria of non-apoptotic cells. Unexpectedly, the substitution of the Bcl-x(L) membrane anchor by the corresponding Bax segment reverses the Bax retrotranslocation activity of Bcl-x(L), but not that of Bcl-x(L) shuttling. Bax retrotranslocation depends on interaction to the Bcl-x(L) membrane anchor and interaction between the Bax BH3 domain and the Bcl-x(L) hydrophobic cleft. Interference with either interaction increases mitochondrial levels of endogenous Bax. In healthy cells, mitochondrial Bax does not permeabilize the outer mitochondrial membrane, but increases cell death after apoptosis induction.
Figures


References
-
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

