Role of km23-1 in RhoA/actin-based cell migration
- PMID: 23079622
- PMCID: PMC3513371
- DOI: 10.1016/j.bbrc.2012.10.047
Role of km23-1 in RhoA/actin-based cell migration
Abstract
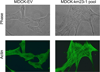
km23-1 was originally identified as a TGFß receptor-interacting protein that plays an important role in TGFß signaling. Moreover, km23-1 is actually part of an ancient superfamily of NTPase-regulatory proteins, widely represented in archaea and bacteria. To further elucidate the function of km23-1, we identified novel protein interacting partners for km23-1 by using tandem affinity purification (TAP) and tandem mass spectrometry (MS). Here we show that km23-1 interacted with a class of proteins involved in actin-based cell motility and modulation of the actin cytoskeleton. We further showed that km23-1 modulates the formation of a highly organized stress fiber network. More significantly, we demonstrated that knockdown (KD) of km23-1 decreased RhoA activation in Mv1Lu epithelial cells. Finally, our results demonstrated for the first time that depletion of km23-1 inhibited cell migration of human colon carcinoma cells (HCCCs) in wound-healing assays. Overall, our findings demonstrate that km23-1 regulates RhoA and motility-associated actin modulating proteins, suggesting that km23-1 may represent a novel target for anti-metastatic therapy.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Spano D, Heck C, De Antonellis P, Christofori G, Zollo M. Molecular networks that regulate cancer metastasis. Semin Cancer Biol. 2012;22:234–249. - PubMed
-
- Bailly M, Condeelis J. Cell motility: insights from the backstage. Nat Cell Biol. 2002;4:E292–E294. - PubMed
-
- Le Clainche C, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiological reviews. 2008;88:489–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

