Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin
- PMID: 23079659
- PMCID: PMC3480666
- DOI: 10.1016/j.ccr.2012.08.017
Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin
Abstract
Thymic Stromal Lymphopoietin (TSLP), a cytokine implicated in induction of T helper 2 (Th2)-mediated allergic inflammation, has recently been shown to stimulate solid tumor growth and metastasis. Conversely, studying mice with clonal loss of Notch signaling in their skin revealed that high levels of TSLP released by barrier-defective skin caused a severe inflammation, resulting in gradual elimination of Notch-deficient epidermal clones and resistance to skin tumorigenesis. We found CD4(+) T cells to be both required and sufficient to mediate these effects of TSLP. Importantly, TSLP overexpression in wild-type skin also caused resistance to tumorigenesis, confirming that TSLP functions as a tumor suppressor in the skin.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

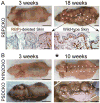
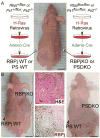
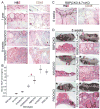


Comment in
-
Tumour immunology: Context is key for TSLP.Nat Rev Cancer. 2012 Dec;12(12):796. doi: 10.1038/nrc3405. Nat Rev Cancer. 2012. PMID: 23175117 No abstract available.
References
-
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. - PubMed
-
- Cario G, Zimmermann M, Romey R, Gesk S, Vater I, Harbott J, Schrauder A, Moericke A, Izraeli S, Akasaka T, et al. Presence of the P2RY8-CRLF2 rearrangement is associated with a poor prognosis in non-high-risk precursor B-cell acute lymphoblastic leukemia in children treated according to the ALL-BFM 2000 protocol. Blood. 2010;115:5393–5397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

