MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles
- PMID: 23080337
- PMCID: PMC6813819
- DOI: 10.1038/nrd3864
MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles
Abstract
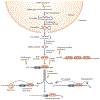
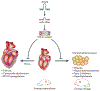
In recent years, prominent roles for microRNAs (miRNAs) have been uncovered in several cardiovascular disorders. The ability to therapeutically manipulate miRNA expression and function through systemic or local delivery of miRNA inhibitors, referred to as antimiRs, has triggered enthusiasm for miRNAs as novel therapeutic targets. Here, we focus on the pharmacokinetic and pharmacodynamic properties of current antimiR designs and their relevance to cardiovascular indications, and evaluate the opportunities and obstacles associated with this new therapeutic modality.
Conflict of interest statement
Competing interests statement
The authors declare
Figures


References
-
- van Rooij E et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl Acad. Sci. USA 103, 18255–18260 (2006). - PMC - PubMed
-
This is the first description of changes in miRNA levels in murine and human heart disorders, and provides evidence for the relevance of miRNAs in heart disease.
-
- Ikeda S et al. Altered microRNA expression in human heart disease. Physiol. Genomics 31, 367–373 (2007). - PubMed
-
This study describes unique miRNA signature patterns for different forms of human heart disease.
-
- Thum T et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 116, 258–267 (2007). - PubMed
-
This study suggests a relationship between changes in miRNAs and the expression of fetal genes in human heart disease.
-
- Bonauer A, Boon RA & Dimmeler S Vascular microRNAs. Curr. Drug Targets 11, 943–949 (2010). - PubMed
-
- Zampetaki A & Mayr M MicroRNAs in vascular and metabolic disease. Circ. Res 110, 508–522 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

