Functional dissection of Pax3 in paraxial mesoderm development and myogenesis
- PMID: 23081715
- PMCID: PMC3528848
- DOI: 10.1002/stem.1254
Functional dissection of Pax3 in paraxial mesoderm development and myogenesis
Abstract
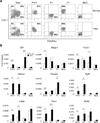
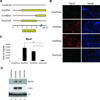
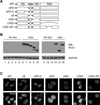
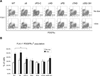
The paired box transcription factor Pax3 is well-known as a major regulator of embryonic myogenesis. Before Pax3 expression becomes restricted to the dermomyotome, this transcription factor is also expressed in the developing somites. The role of Pax3 at this early stage is unclear, in particular because of the scarce frequency of Pax3-positive cells in the early mouse embryo. Inducible gene expression in embryonic stem cells (ESCs) represents an excellent tool to overcome this limitation, since it can provide large quantities of otherwise rare embryonic populations expressing a factor of interest. Here we used engineered mouse ESCs to perform a functional analysis of Pax3 with the aim to identify the molecular determinants involved in the early functions of this transcription factor. We find that Pax3 induction during embryoid body differentiation results in the upregulation of genes expressed in the presomitic and somitic mesoderm. Moreover, we show that paraxial mesoderm induced by transient expression of Pax3 is not irreversibly committed to myogenesis rather requires sustained Pax3 expression. Using a series of deletion mutants of Pax3, which differentially affect its transcriptional activity, we map protein domains necessary for induction of paraxial mesoderm and induction of the myogenic program. The paired, homeo-, and transcriptional activation domains were each required for both processes, however, the paired-c-terminal RED domain showed a paraxial mesoderm-specific activity that was dispensable for myogenesis. These findings demonstrate and provide mechanistic insight into an early role for Pax3 in the generation of paraxial mesoderm.
Copyright © 2012 AlphaMed Press.
Figures


References
-
- Tam PP, Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. - PubMed
-
- Brand-Saberi B, Wilting J, Ebensperger C, et al. The formation of somite compartments in the avian embryo. Int J Dev Biol. 1996;40:411–420. - PubMed
-
- Buckingham M, Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol. 2007;23:645–673. - PubMed
-
- Epstein DJ, Vekemans M, Gros P. Splotch (Sp2H), a mutation affecting development of the mouse neural tube, shows a deletion within the paired homeodomain of Pax-3. Cell. 1991;67:767–774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

