Effect of heat-killed Escherichia coli, lipopolysaccharide, and muramyl dipeptide treatments on the immune response phenotype and allergy in neonatal pigs sensitized to the egg white protein ovomucoid
- PMID: 23081818
- PMCID: PMC3535875
- DOI: 10.1128/CVI.00555-12
Effect of heat-killed Escherichia coli, lipopolysaccharide, and muramyl dipeptide treatments on the immune response phenotype and allergy in neonatal pigs sensitized to the egg white protein ovomucoid
Abstract
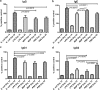
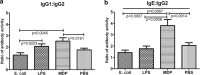
Predisposition to food allergies may reflect a type 2 immune response (IR) bias in neonates due to the intrauterine environment required to maintain pregnancy. The hygiene hypothesis states that lack of early environmental stimulus leading to inappropriate development and bias in IR may also contribute. Here, the ability of heat-killed Escherichia coli, lipopolysaccharide (LPS), or muramyl dipeptide (MDP) to alter IR bias and subsequent allergic response in neonatal pigs was investigated. Three groups of three litters of pigs (12 pigs/litter) were given intramuscular injections of E. coli, LPS, MDP, or phosphate-buffered saline (PBS) (control) and subsequently sensitized to the egg white allergen ovomucoid using an established protocol. To evaluate change in IR bias, immunoglobulin isotype-associated antibody activity (AbA), concentrations of type 1 and 2 and proinflammatory cytokines released from mitogen-stimulated blood mononuclear cells, and the percentage of T-regulatory cells (T-regs) in blood were measured. Clinical signs of allergy were assessed after oral challenge with egg white. The greatest effect on IR bias was observed in MDP-treated pigs, which had a type 2-biased phenotype by isotype-specific AbA, cytokine production, and a low proportion of T-regs. LPS-treated pigs had decreased type 1- and type 2-associated AbA. E. coli-treated pigs displayed increased response to Ovm as AbA and had more balanced cytokine profiles, as well as the highest proportion of T-regs. Accordingly, pigs treated with MDP were more susceptible to allergy than PBS controls, while pigs treated with LPS were less susceptible. Treatment with E. coli did not significantly alter the frequency of clinical signs.
Figures



Similar articles
-
Immune response phenotype of allergic versus clinically tolerant pigs in a neonatal swine model of allergy.Vet Immunol Immunopathol. 2013 Jul 15;154(1-2):17-24. doi: 10.1016/j.vetimm.2013.04.008. Epub 2013 Apr 16. Vet Immunol Immunopathol. 2013. PMID: 23664639
-
Attenuation of allergy to ovomucoid in pigs by neonatal treatment with heat-killed Escherichia coli or E. coli producing porcine IFN-gamma.Vet Immunol Immunopathol. 2009 Nov 15;132(1):78-83. doi: 10.1016/j.vetimm.2009.09.019. Epub 2009 Sep 24. Vet Immunol Immunopathol. 2009. PMID: 19833393
-
A neonatal swine model of allergy induced by the major food allergen chicken ovomucoid (Gal d 1).Int Arch Allergy Immunol. 2008;146(1):11-8. doi: 10.1159/000112498. Epub 2007 Dec 14. Int Arch Allergy Immunol. 2008. PMID: 18087157
-
Practical immunoregulation: neonatal immune response variation and prophylaxis of experimental food allergy in pigs.Vet Immunol Immunopathol. 2012 Jul 15;148(1-2):110-5. doi: 10.1016/j.vetimm.2011.03.010. Epub 2011 Mar 12. Vet Immunol Immunopathol. 2012. PMID: 21489640 Review.
-
Role of Muramyl Dipeptide in Lipopolysaccharide-Mediated Biological Activity and Osteoclast Activity.Anal Cell Pathol (Amst). 2018 Feb 14;2018:8047610. doi: 10.1155/2018/8047610. eCollection 2018. Anal Cell Pathol (Amst). 2018. PMID: 29666781 Free PMC article. Review.
Cited by
-
A Single-Dose Intramuscular Nanoparticle Vaccine With or Without Prior Intrauterine Priming Triggers Specific Uterine and Colostral Mucosal Antibodies and Systemic Immunity in Gilts but Not Passive Protection for Suckling Piglets.Front Vet Sci. 2022 Aug 3;9:931232. doi: 10.3389/fvets.2022.931232. eCollection 2022. Front Vet Sci. 2022. PMID: 35990278 Free PMC article.
References
-
- Akdis CA, Akdis M. 2009. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J. Allergy Clin. Immunol. 123: 735– 746 - PubMed
-
- Akdis M, Akdis CA. 2009. Therapeutic manipulation of immune tolerance in allergic disease. Nat. Rev. Drug Discov. 8: 645– 660 - PubMed
-
- Allen CW, Campbell DE, Kemp AS. 2009. Food allergy: is strict avoidance the only answer? Pediatr. Allergy Immunol. 20: 415– 422 - PubMed
-
- Butler JE, Francis DH, Freeling J, Weber P, Krieg AM. 2005. Piglets. IX. Three pathogen-associated molecular patterns act synergistically to allow germfree piglets to respond to type 2 thymus-independent and thymus-dependent antigens. J. Immunol. 4: 6772– 6785 - PubMed
-
- Butler JE, Wertz N. 2006. Antibody repertoire development in fetal and neonatal piglets. XVII. IgG subclass transcription revisited with emphasis on new IgG3. J. Immunol. 177: 5480– 5489 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

