Aging promotes the development of diet-induced murine steatohepatitis but not steatosis
- PMID: 23081825
- PMCID: PMC3566282
- DOI: 10.1002/hep.26099
Aging promotes the development of diet-induced murine steatohepatitis but not steatosis
Abstract
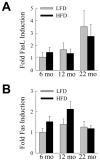
The prevalence of the metabolic syndrome and nonalcoholic fatty liver disease (NAFLD) in humans increases with age. It is unknown whether this association is secondary to the increased incidence of risk factors for NAFLD that occurs with aging, reflects the culmination of years of exposure to lifestyle factors such as a high-fat diet (HFD), or results from physiological changes that characterize aging. To examine this question, the development of NAFLD in response to a fixed period of HFD feeding was examined in mice of different ages. Mice aged 2, 8, and 18 months were fed 16 weeks of a low-fat diet or HFD. Increased body mass and insulin insensitivity occurred in response to HFD feeding irrespective of the age of the mice. The amount of HFD-induced hepatic steatosis as determined biochemically and histologically was also equivalent among the three ages. Liver injury occurred exclusively in the two older ages as reflected by increased serum alanine aminotransferase levels, positive terminal deoxynucleotide transferase-mediated deoxyuridine triphosphate nick end-labeling, and caspase activation. Older mice also had an elevated innate immune response with a more pronounced polarization of liver and adipose tissue macrophages into an M1 phenotype. Studies of cultured hepatocytes from young and old mice revealed that aged cells were selectively sensitized to the Fas death pathway.
Conclusion: Aging does not promote the development of hepatic steatosis but leads to increased hepatocellular injury and inflammation that may be due in part to sensitization to the Fas death pathway and increased M1 macrophage polarization.
Copyright © 2012 American Association for the Study of Liver Diseases.
Figures
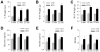



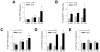
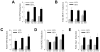
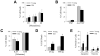
Comment in
-
Reply: To PMID 23081825.Hepatology. 2013 Aug;58(2):831. doi: 10.1002/hep.26214. Epub 2013 Jun 24. Hepatology. 2013. PMID: 23280403 No abstract available.
-
Old age and steatohepatitis: a dangerous liaison?Hepatology. 2013 Aug;58(2):830-1. doi: 10.1002/hep.26212. Epub 2013 Jun 12. Hepatology. 2013. PMID: 23281079 No abstract available.
References
-
- Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171. - PubMed
-
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. - PubMed
-
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. - PubMed
-
- Lee JY, Kim KM, Lee SG, Yu E, Lim YS, Lee HC, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol. 2007;47:239–244. - PubMed
-
- Amarapurkar D, Kamani P, Patel N, Gupte P, Kumar P, Agal S, et al. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6:161–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
