Protective effect of carnosic acid, a pro-electrophilic compound, in models of oxidative stress and light-induced retinal degeneration
- PMID: 23081978
- PMCID: PMC3508754
- DOI: 10.1167/iovs.12-10793
Protective effect of carnosic acid, a pro-electrophilic compound, in models of oxidative stress and light-induced retinal degeneration
Abstract
Purpose: The herb rosemary has been reported to have antioxidant and anti-inflammatory activity. We have previously shown that carnosic acid (CA), present in rosemary extract, crosses the blood-brain barrier to exert neuroprotective effects by upregulating endogenous antioxidant enzymes via the Nrf2 transcriptional pathway. Here we investigated the antioxidant and neuroprotective activity of CA in retinal cell lines exposed to oxidative stress and in a rat model of light-induced retinal degeneration (LIRD).
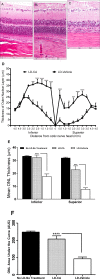
Methods: Retina-derived cell lines ARPE-19 and 661W treated with hydrogen peroxide were used as in vitro models for testing the protective activity of CA. For in vivo testing, dark-adapted rats were given intraperitoneal injections of CA prior to exposure to white light to assess protection of the photoreceptor cells. Retinal damage was assessed by measuring outer nuclear layer thickness and by electroretinogram (ERG).
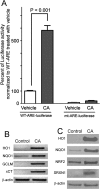
Results: In vitro, CA significantly protected retina-derived cell lines (ARPE-19 and 661W) against H(2)O(2)-induced toxicity. CA induced antioxidant phase 2 enzymes and reduced formation of hyperoxidized peroxiredoxin (Prx)2. Similarly, we found that CA protected retinas in vivo from LIRD, producing significant improvement in outer nuclear layer thickness and ERG activity.
Conclusions: These findings suggest that CA may potentially have clinical application to diseases affecting the outer retina, including age-related macular degeneration and retinitis pigmentosa, in which oxidative stress is thought to contribute to disease progression.
Conflict of interest statement
Disclosure:
Figures



References
-
- Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134 - PubMed
-
- Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

