Ethacrynic acid and a derivative enhance apoptosis in arsenic trioxide-treated myeloid leukemia and lymphoma cells: the role of glutathione S-transferase p1-1
- PMID: 23082001
- PMCID: PMC3525753
- DOI: 10.1158/1078-0432.CCR-12-0770
Ethacrynic acid and a derivative enhance apoptosis in arsenic trioxide-treated myeloid leukemia and lymphoma cells: the role of glutathione S-transferase p1-1
Abstract
Purpose: Arsenic trioxide (ATO) as a single agent is used for treatment of acute promyelocytic leukemia (APL) with minimal toxicity, but therapeutic effect of ATO in other types of malignancies has not been achieved. We tested whether a combination with ethacrynic acid (EA), a glutathione S-transferase P1-1 (GSTP1-1) inhibitor, and a reactive oxygen species (ROS) inducer will extend the therapeutic effect of ATO beyond APL.
Experimental design: The combined apoptotic effects of ATO plus ethacrynic acid were tested in non-APL leukemia and lymphoma cell lines. The role of ROS, GSTP1-1, glutathione (GSH), and Mcl-1 in apoptosis was determined. The selective response to this combination of cells with and without GSTP1-1 expression was compared.
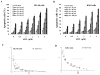
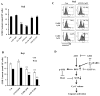
Results: ATO/EA combination synergistically induced apoptosis in myeloid leukemia and lymphoma cells. This treatment produced high ROS levels, activated c-jun-NH(2)-kinase (JNK), and reduced Mcl-1 protein. This led to the decrease of mitochondrial transmembrane potential, release of cytochrome c, and subsequently, to activation of caspase-3 and -9. Induction of apoptosis in leukemia and lymphoma cells expressing GSTP1-1 required high ethacrynic acid concentrations to be combined with ATO. Silencing of GSTP1 in leukemia cells sensitized them to ATO/EA-induced apoptosis. In a subgroup of B-cell lymphoma, which does not express GSTP1-1, lower concentrations of ethacrynic acid and its more potent derivative, ethacrynic acid butyl-ester (EABE), decreased intracellular GSH levels and synergistically induced apoptosis when combined with ATO.
Conclusion: B-cell lymphoma cells lacking GSTP1-1 are more sensitive than myeloid leukemia cells to ATO/EA-induced apoptosis.
©2012 AACR.
Conflict of interest statement
Figures




References
-
- Niu C, Yan H, Yu T, Sun HP, Liu JX, Li XS, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94:3315–24. - PubMed
-
- Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–60. - PubMed
-
- Jing Y, Wang L, Xia L, Chen GQ, Chen Z, Miller WH, et al. Combined effect of all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemiacells in vitro and in vivo. Blood. 2001;97:264–9. - PubMed
-
- Chen GQ, Zhou L, Styblo M, Walton F, Jing Y, Weinberg R, et al. Methylated metabolites of arsenic trioxide are more potent than arsenic trioxide as apoptotic but not differentiation inducers in leukemia and lymphoma cells. Cancer Res. 2003;63:1853–9. - PubMed
-
- Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93:268–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

