Effect of molecular organization on the image histograms of polarization SHG microscopy
- PMID: 23082306
- PMCID: PMC3470008
- DOI: 10.1364/BOE.3.002681
Effect of molecular organization on the image histograms of polarization SHG microscopy
Abstract
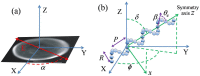
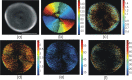
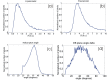
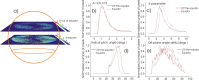
Based on its polarization dependency, second harmonic generation (PSHG) microscopy has been proven capable to structurally characterize molecular architectures in different biological samples. By exploiting this polarization dependency of the SHG signal in every pixel of the image, average quantitative structural information can be retrieved in the form of PSHG image histograms. In the present study we experimentally show how the PSHG image histograms can be affected by the organization of the SHG active molecules. Our experimental scenario grounds on two inherent properties of starch granules. Firstly, we take advantage of the radial organization of amylopectin molecules (the SHG source in starch) to attribute shifts of the image histograms to the existence of tilted off the plane molecules. Secondly, we use the property of starch to organize upon hydration to demonstrate that the degree of structural order at the molecular level affects the width of the PSHG image histograms. The shorter the width is the more organized the molecules in the sample are, resulting in a reliable method to measure order. The implication of this finding is crucial to the interpretation of PSHG images used for example in tissue diagnostics.
Keywords: (180.4315) Nonlinear microscopy; (190.2620) Harmonic generation and mixing.
Figures
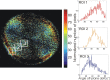
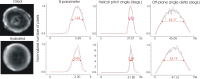
Similar articles
-
Comparison of Different Polarization Sensitive Second Harmonic Generation Imaging Techniques.Methods Protoc. 2019 Jun 7;2(2):49. doi: 10.3390/mps2020049. Methods Protoc. 2019. PMID: 31181703 Free PMC article.
-
PSHG-TISS: A collection of polarization-resolved second harmonic generation microscopy images of fixed tissues.Sci Data. 2022 Jul 2;9(1):376. doi: 10.1038/s41597-022-01477-1. Sci Data. 2022. PMID: 35780180 Free PMC article.
-
Polarization Second Harmonic Generation Discriminates Between Fresh and Aged Starch-Based Adhesives Used in Cultural Heritage.Microsc Microanal. 2016 Oct;22(5):1072-1083. doi: 10.1017/S1431927616011570. Epub 2016 Sep 13. Microsc Microanal. 2016. PMID: 27619334
-
Advanced microscopy techniques for revealing molecular structure of starch granules.Biophys Rev. 2020 Feb;12(1):105-122. doi: 10.1007/s12551-020-00614-7. Epub 2020 Jan 16. Biophys Rev. 2020. PMID: 31950343 Free PMC article. Review.
-
Protein conformation and molecular order probed by second-harmonic-generation microscopy.J Biomed Opt. 2012 Jun;17(6):060901. doi: 10.1117/1.JBO.17.6.060901. J Biomed Opt. 2012. PMID: 22734730 Review.
Cited by
-
Multiscale investigation of collagen structure in human skin and gel matrices using polarization resolved second harmonic generation microscopy.Sci Rep. 2025 Jun 6;15(1):20025. doi: 10.1038/s41598-025-02536-4. Sci Rep. 2025. PMID: 40481016 Free PMC article.
-
Translational label-free nonlinear imaging biomarkers to classify the human corneal microstructure.Biomed Opt Express. 2015 Jul 8;6(8):2803-18. doi: 10.1364/BOE.6.002803. eCollection 2015 Aug 1. Biomed Opt Express. 2015. PMID: 26309745 Free PMC article.
-
Polarimetric second-harmonic generation microscopy of partially oriented fibers I: Digital modeling.Biophys J. 2023 Oct 3;122(19):3924-3936. doi: 10.1016/j.bpj.2023.08.016. Epub 2023 Aug 22. Biophys J. 2023. PMID: 37608550 Free PMC article.
-
Stokes polarimetry-based second harmonic generation microscopy for collagen and skeletal muscle fiber characterization.Lasers Med Sci. 2021 Aug;36(6):1161-1167. doi: 10.1007/s10103-020-03144-6. Epub 2020 Sep 18. Lasers Med Sci. 2021. PMID: 32945997 Free PMC article.
-
Three-dimensional characterization of collagen remodeling in cell-seeded collagen scaffolds via polarization second harmonic generation.Biomed Opt Express. 2021 Jan 28;12(2):1136-1153. doi: 10.1364/BOE.411501. eCollection 2021 Feb 1. Biomed Opt Express. 2021. PMID: 33680563 Free PMC article.
References
-
- Psilodimitrakopoulos S., Santos S. I., Amat-Roldan I., Thayil A. K., Artigas D., Loza-Alvarez P., “In vivo, pixel-resolution mapping of thick filaments’ orientation in nonfibrilar muscle using polarization-sensitive second harmonic generation microscopy,” J. Biomed. Opt. 14(1), 014001 (2009).10.1117/1.3059627 - DOI - PubMed
LinkOut - more resources
Full Text Sources
