Ketone body utilization drives tumor growth and metastasis
- PMID: 23082722
- PMCID: PMC3507492
- DOI: 10.4161/cc.22137
Ketone body utilization drives tumor growth and metastasis
Abstract
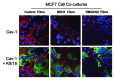
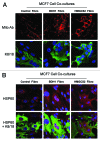
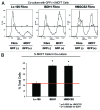
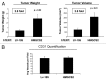
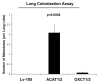
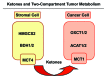
We have previously proposed that catabolic fibroblasts generate mitochondrial fuels (such as ketone bodies) to promote the anabolic growth of human cancer cells and their metastasic dissemination. We have termed this new paradigm "two-compartment tumor metabolism." Here, we further tested this hypothesis by using a genetic approach. For this purpose, we generated hTERT-immortalized fibroblasts overexpressing the rate-limiting enzymes that promote ketone body production, namely BDH1 and HMGCS2. Similarly, we generated MDA-MB-231 human breast cancer cells overexpressing the key enzyme(s) that allow ketone body re-utilization, OXCT1/2 and ACAT1/2. Interestingly, our results directly show that ketogenic fibroblasts are catabolic and undergo autophagy, with a loss of caveolin-1 (Cav-1) protein expression. Moreover, ketogenic fibroblasts increase the mitochondrial mass and growth of adjacent breast cancer cells. However, most importantly, ketogenic fibroblasts also effectively promote tumor growth, without a significant increase in tumor angiogenesis. Finally, MDA-MB-231 cells overexpressing the enzyme(s) required for ketone re-utilization show dramatic increases in tumor growth and metastatic capacity. Our data provide the necessary genetic evidence that ketone body production and re-utilization drive tumor progression and metastasis. As such, ketone inhibitors should be designed as novel therapeutics to effectively treat advanced cancer patients, with tumor recurrence and metastatic disease. In summary, ketone bodies behave as onco-metabolites, and we directly show that the enzymes HMGCS2, ACAT1/2 and OXCT1/2 are bona fide metabolic oncogenes.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
